Oral sedative for children
A sedative and children's technology, applied in the direction of medical preparations of non-active ingredients, drug delivery, active ingredients of hydroxyl compounds, etc., can solve problems such as unpredictable vomiting, vomiting, and physical injury of children
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 2
[0014] Implementation method 2: select mass concentration as 15% chloral hydrate solution 20ml, simple syrup 15ml, mass concentration 10% glucose 10ml, sodium citrate 800mg, after sodium citrate 800mg is dissolved in 10% glucose solution earlier, 20ml Chloral hydrate solution, simple syrup 15ml, and glucose dissolved in sodium citrate were mixed, and the mixed solution was placed in a drug shaker and shaken at room temperature for 4 hours to form a solution with a pH value of 7.5, and the oral sedative was completed. configuration.
Embodiment approach 3
[0015] Implementation method 3: select mass concentration as 20% chloral hydrate solution 20ml, simple syrup 20ml, mass concentration 5% glucose 10ml, sodium citrate 800mg, after sodium citrate 800mg is dissolved in 5% glucose solution earlier, 20ml Chloral hydrate solution, simple syrup 20ml, and glucose dissolved in sodium citrate were mixed, and the mixed solution was placed in a drug shaker and shaken at room temperature for 4 hours to form a solution with a pH value of 7.8, and the oral sedative was completed. configuration.
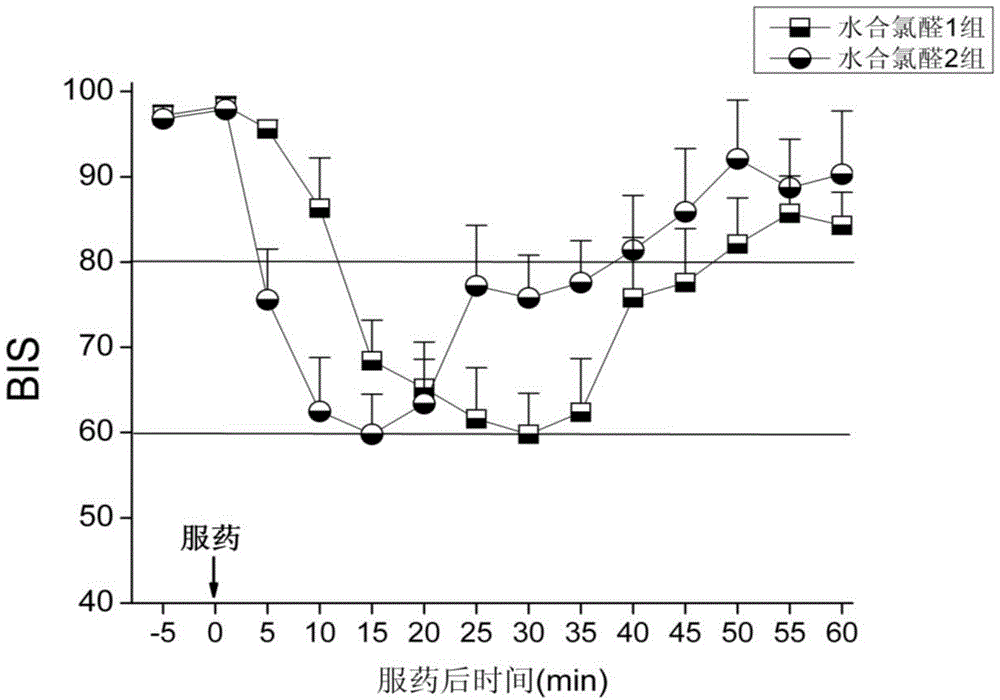
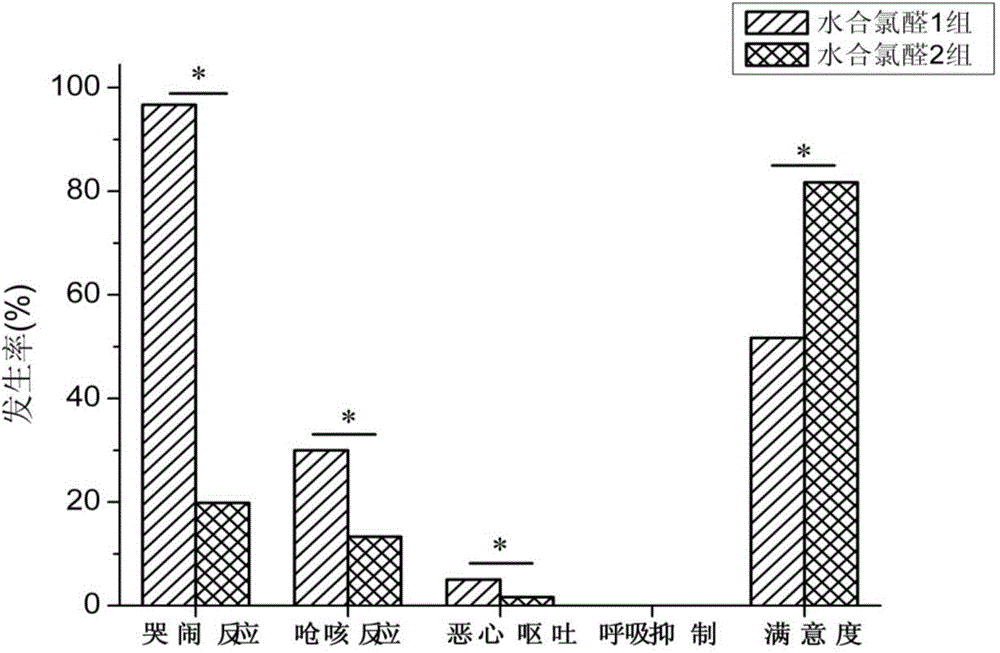
[0016] The oral sedatives for children configured above were tested after all participating children were reviewed and approved by the hospital ethics committee, and their families signed the informed consent. A total of 120 children who needed to be sedated for examination were selected, ASA Ⅰ-Ⅱ grade, aged 1 month to 3 years old, and those with a history of allergy to chloral hydrate and severe liver and kidney dysfunction were excluded from the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com