Stable chloral hydrate syrup as well as preparation method, quality control method and application thereof
A technology of chloral hydrate syrup and chloral hydrate, which is applied in the field of medicine, can solve the problems of poor stability, harsh storage conditions, and short validity period of chloral hydrate, and achieve the effects of accurate dosage, scientific design, and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
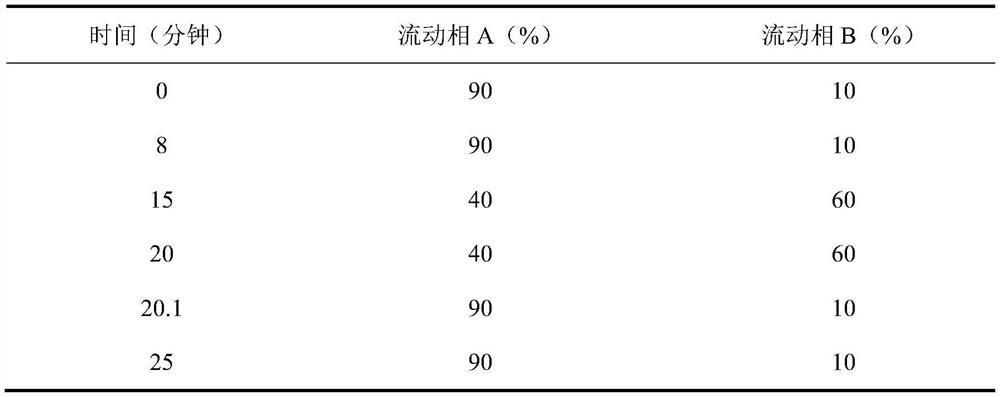
[0064] Preparation of reference solution: Accurately weigh an appropriate amount of 5-hydroxymethylfurfural reference substance, dissolve it with 10mmol / L potassium dihydrogen phosphate solution (adjust the pH value to 4.0 with phosphoric acid)-acetonitrile (85:15) to make a concentration of 5.0 μg / ml of the reference substance solution;
[0065] Preparation of the test solution: get an appropriate amount of chloral hydrate syrup test sample, accurately weighed, dissolve or dilute and constant volume with mobile phase, to obtain a test solution with a chloral hydrate concentration of about 1 mg / ml;
[0066] Get each 10 μ L of need testing solution, reference substance solution, inject liquid chromatograph respectively, measure according to described chromatographic conditions, record chromatogram, according to the chromatogram of need testing solution and the chromatogram of reference substance solution, determine the The content of 5-hydroxymethylfurfural in the product solu...
Embodiment 1
[0076] The present embodiment discloses the prescription of chloral hydrate syrup of the present invention and preparation method thereof, and prescription is:
[0077]
[0078] The preparation method is as follows: respectively take prescription quantities of sodium benzoate and stevioside, add appropriate amount of purified water respectively, stir until dissolved, and prepare sodium benzoate solution and stevioside solution. Weigh the prescribed amount of chloral hydrate, add an equal amount of 0.1M hydrochloric acid solution, stir until completely dissolved, and prepare a chloral hydrate solution.
[0079] Preparation of syrup: Weigh 50g of sucrose and 12g of purified water in a beaker, heat and boil for 10min, when the temperature drops to about 50°C, add the prescribed amount of glycerin, stevia solution, sodium benzoate solution, stir for 15min, cool down to about 25°C, add Stir and mix the chloral hydrate solution, adjust the pH value to 2.0 with hydrochloric acid s...
Embodiment 2
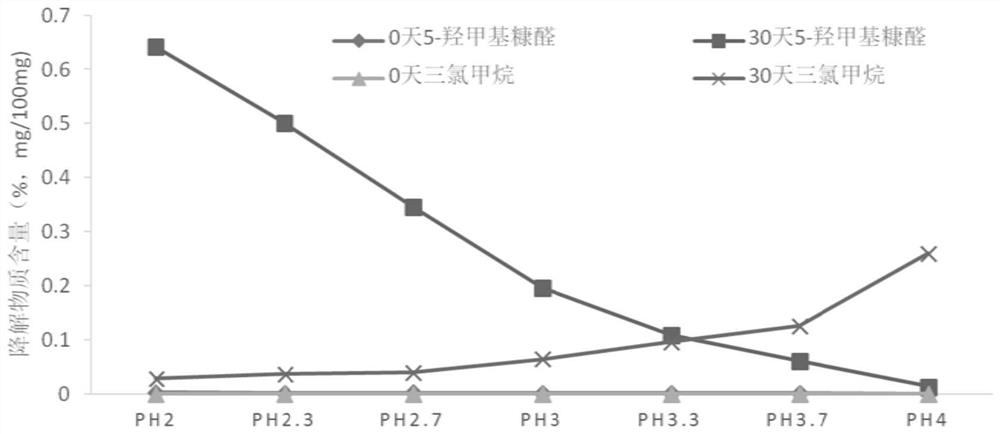
[0081] This example discloses the investigation of different pHs of chloral hydrate syrup. The preparation method of the chloral hydrate syrup described in this embodiment is the same as that of Example 1, the only difference being that the final adjusted pH value is different. . The prepared samples with different pH values were placed at 60°C for 30 days, and the degradation products chloroform and 5-hydroxymethylfurfural were used as indicators to conduct stability investigations. The results are shown in the table below:
[0082] Table 5. Results of Stability Investigation of Chloral Hydrate Syrup with Different pH Values (pH: 2.0~4.0)
[0083]
[0084] From the test results in Table 5, it can be seen that within the range of pH 2.0 to pH 4.0, as the pH increases, the content of the degradation product chloroform in each numbered sample gradually increases. According to the requirements of the residual solvent limit, The control limit of chloroform in the chloral...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com