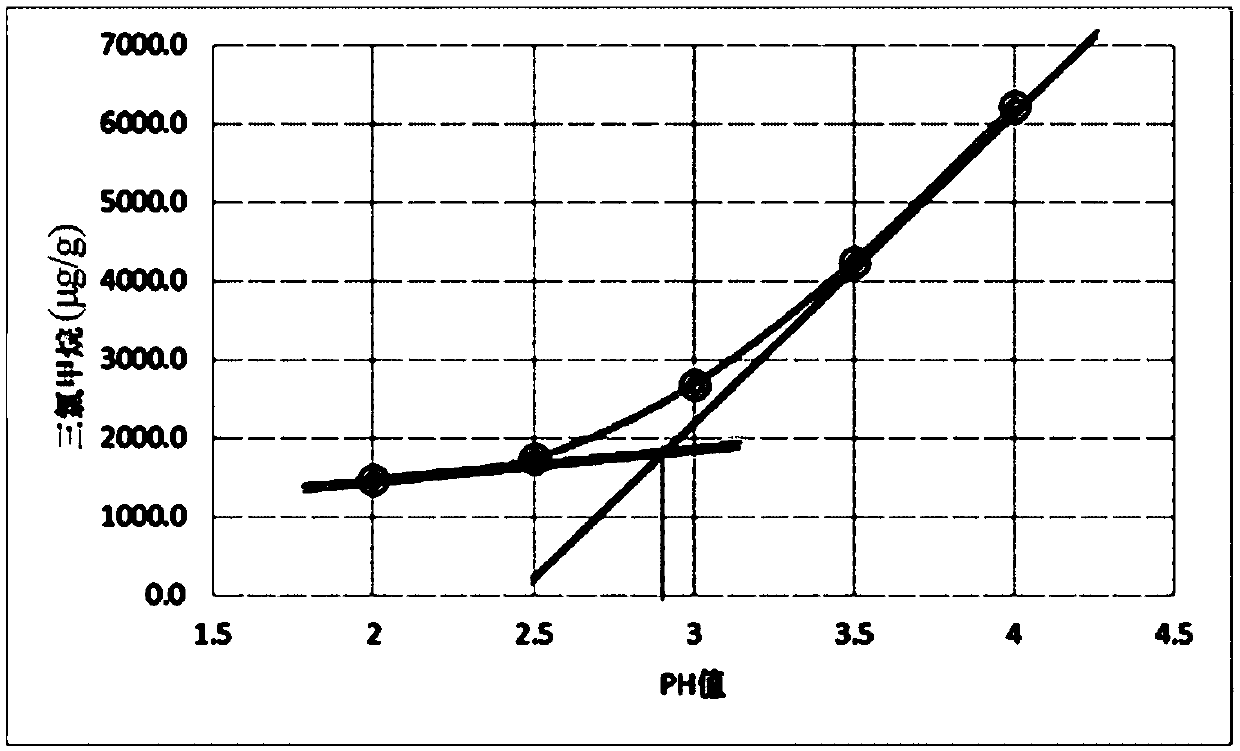
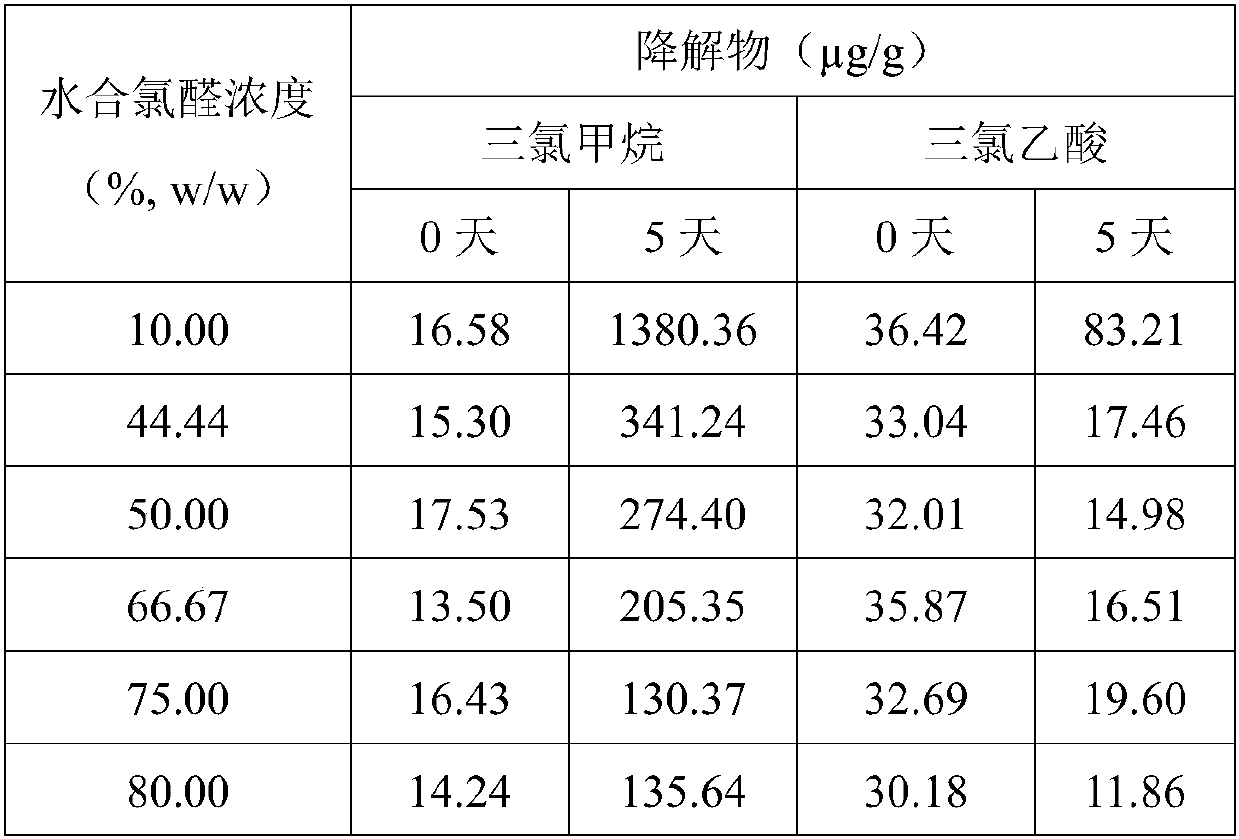
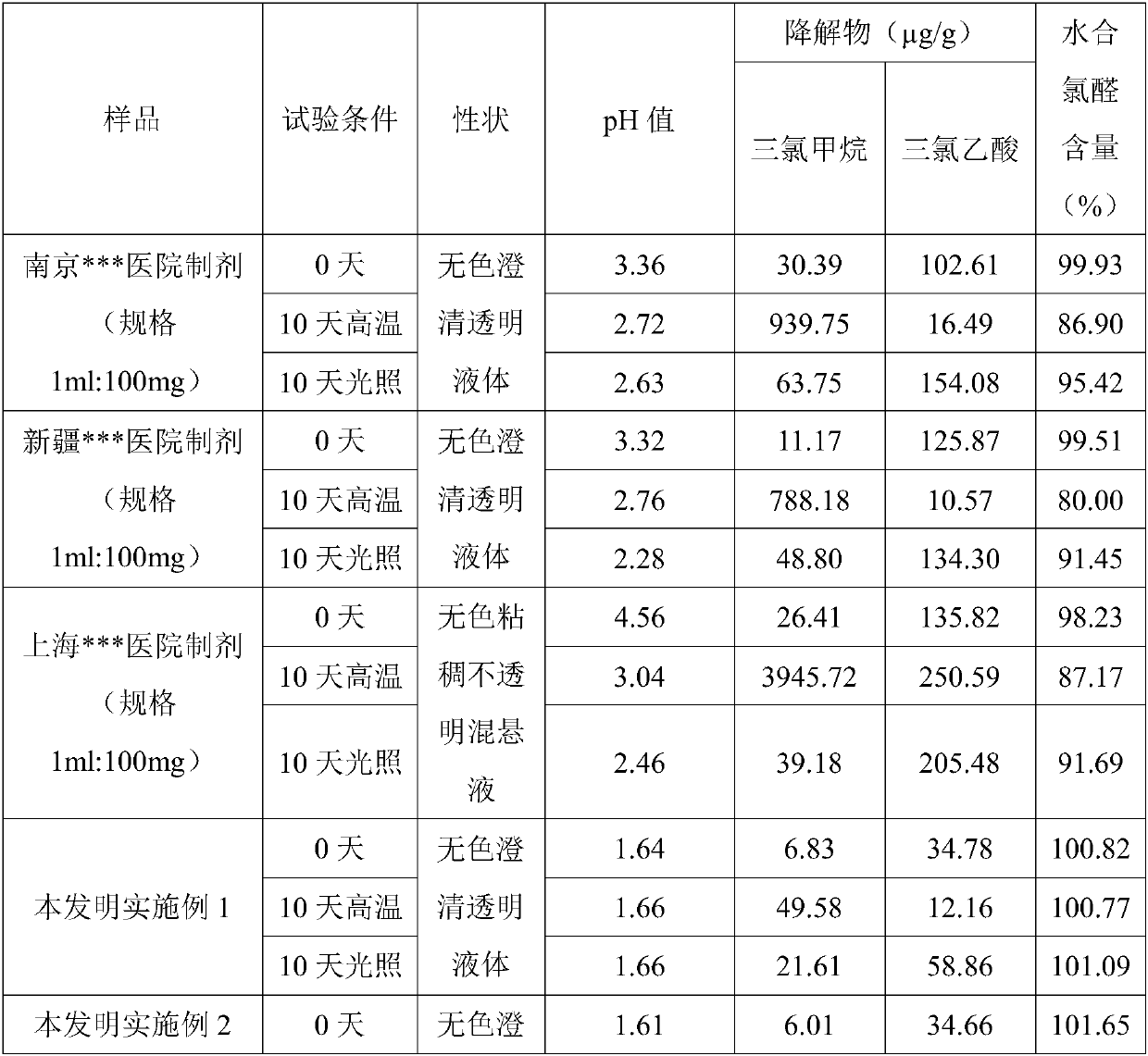
Stable chloral hydrate solution and preparation method and application thereof
A chloral hydrate solution technology, which is applied in the field of chloral hydrate solution and sedative hypnotic drug preparations, can solve problems such as poor compliance, increased children's fear and pain, and large toxic and side effects, and achieve the effect of increasing the concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] In some embodiments, the present invention provides the preparation method of chloral hydrate solution, it is characterized in that: its preparation step comprises:
[0033] 1) mixing a pharmaceutically acceptable acid with water, stirring and dissolving to obtain an acid solution with a pH value of 1.0-2.9; and
[0034] 2) Add chloral hydrate to the acid solution while stirring, and stir until completely dissolved to obtain the chloral hydrate solution, wherein the mass concentration of chloral hydrate is 40%-80% (such as 44%-80% %, 44%-70%, 44%-60% or 44%-50%).
[0035] In a preferred embodiment, the pH of the acid solution prepared in step 1) of the method is 1.0-2.0, preferably 1.2-1.6.
[0036] In a preferred embodiment, the pH of the acid solution prepared in step 1) of the method is 1.31-2.0.
[0037] In a preferred embodiment, the pH of the acid solution prepared in step 1) of the method is 1.61-2.0.
[0038] In other embodiments, the present invention provid...
Embodiment 1
[0056] Add 12g of anhydrous citric acid and a certain amount of purified water into a glass beaker, stir and dissolve to obtain a citric acid solution with a pH value of 1.64, then add 738g of chloral hydrate (14-24 mesh) while stirring, Stir under the rotating speed of / min until dissolving completely, obtain the chloral hydrate solution that mass concentration is 44%.
Embodiment 2
[0058] Add 12g of anhydrous citric acid and a certain amount of purified water into a glass beaker, stir and dissolve to obtain a citric acid solution with a pH value of 1.61, then add 1007g of chloral hydrate (14-24 mesh) while stirring, Stir under the rotating speed of / min until completely dissolving, obtain the chloral hydrate solution that mass concentration is 60%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com