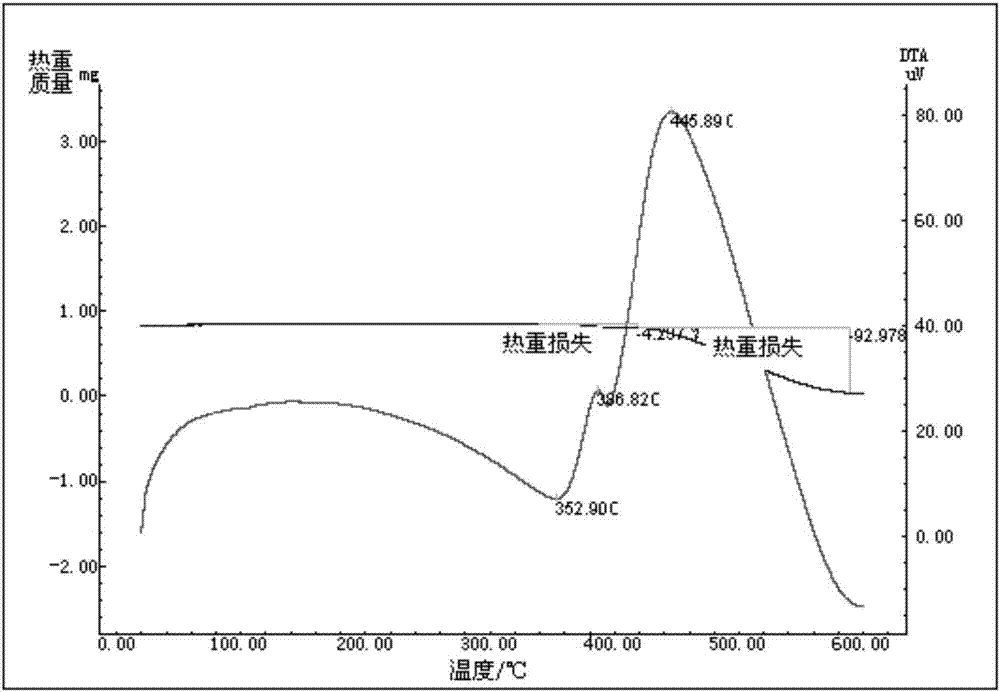
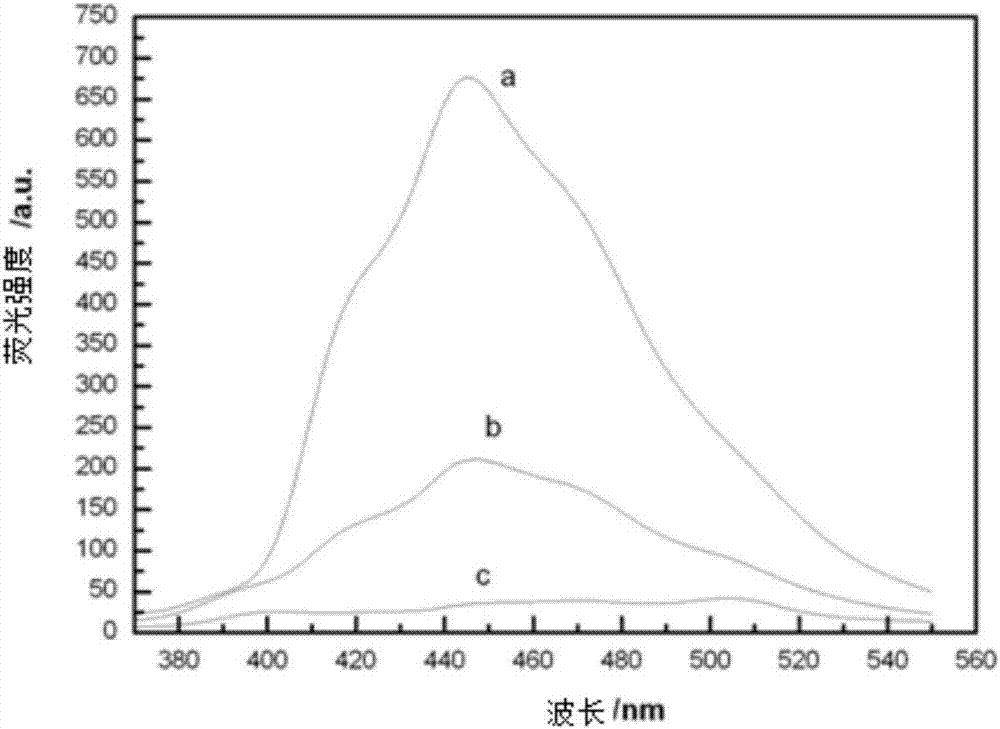
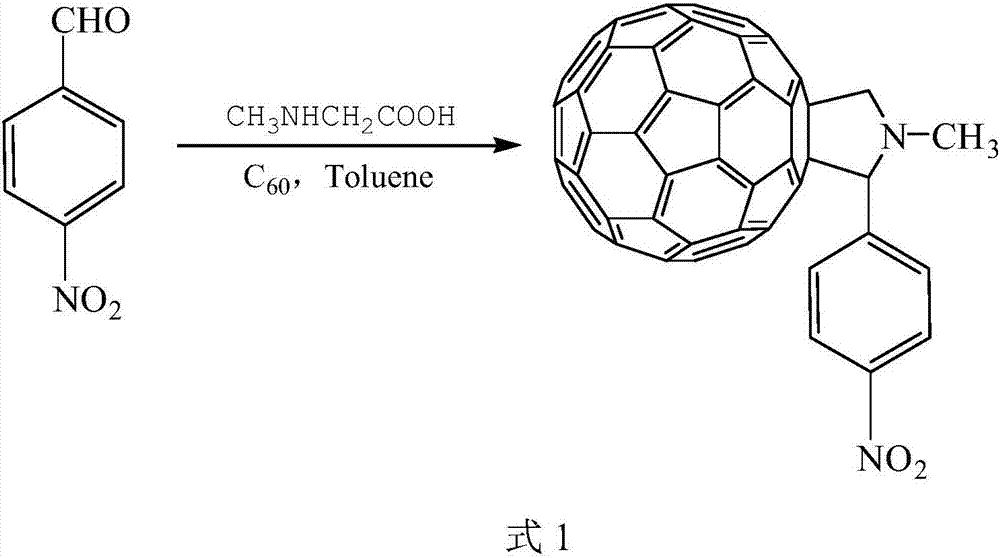
Preparation method of N-methyl-2-(4-nitrophenyl)-3,4-fulleropyrrolidine
A technology of fullerene pyrrolidine and nitrophenyl is applied in the field of preparation of fullerene derivatives, and can solve the problems of low yield, long reaction time, complicated separation steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] (1) According to the principle of the Prato reaction, add 80 mL of freshly distilled toluene solution and 72 mg of C in a 250 mL three-necked flask 60 Powder, continuously feed pure rare gas Ar at room temperature, remove the air in the reaction device to protect the reactants, and when the temperature is 20-25 ° C, magnetic stirring to C 60 The powder was completely dissolved in toluene solution to obtain a purple transparent solution.
[0048] (2) Add 40 mg sarcosine and 45.3 mg recrystallized p-nitrobenzaldehyde respectively, heat in an oil bath at 125-130° C., condense and reflux for 2.5-3 hours.
[0049] During this reaction process, absorb the reaction solution with a capillary glass tube every 30 minutes and spot it on a silica gel plate, and then put the silica gel plate into a mixture of toluene and petroleum ether with a volume ratio of 1:4 (boiling range is 60-90°C ) "climb the plate" in the exhibition tank of the mixed solution for 10-15 minutes, take it ou...
Embodiment 2
[0072] Same as Example 1, except for the following differences:
[0073] C in step (1) 60 Powder is 72mg, toluene is 36mL;
[0074] In step (2), sarcosine is 30mg, p-nitrobenzaldehyde is 15mg, the heating temperature is 120°C, and the reflux time is 4h;
[0075] The drying time in step (5) is 6h.
[0076] The product yield was 28.6%.
[0077] Through the same characterization as the embodiment, the result is attributed to C 60 . The thermal stability and fluorescence properties are comparable to those of the product obtained in Example 1.
Embodiment 3
[0079] Same as Example 1, except for the following differences:
[0080] C in step (1) 60 Powder is 72mg, toluene is 100mL;
[0081] In step (2), sarcosine is 48mg, p-nitrobenzaldehyde is 72mg, the heating temperature is 135°C, and the reflux time is 1h;
[0082] The drying time in step (5) is 24h.
[0083] The product yield was 26.8%.
[0084] Through the same characterization as the embodiment, the result is attributed to C 60 . The thermal stability and fluorescence properties are comparable to those of the product obtained in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com