Preparation method and application of adjustable metal organic cage compound for efficiently selective catalytic reduction of nitrobenzaldehyde
A technology of nitrobenzaldehyde and cage compounds, which is applied in the preparation of organic compounds, iron group organic compounds without C-metal bonds, 2/12 groups of organic compounds without C-metal bonds, etc., can solve environmental pollution , the selectivity needs to be improved, etc., to achieve the effect of low price, high yield and stable chemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1 compound Fe-FPB
[0030] Methyl propargyl (1.68g, 20mmol), benzaldehyde (1.06g, 10mmol) and 2-furylamine (1.13g, 10mmol) were added to 2.0 ml of glacial acetic acid, stirred at 80°C for 20 Hour. The reaction solution was cooled and 25 ml of ethanol was added, sonicated until the solid was powdered, filtered with suction and washed with ethanol to filter the obtained filter cake, and the washed filter cake was vacuum-dried to obtain 1.78 g of a light yellow solid with a yield of 51.8%. 1 H NMR (400MHz, CDCl 3 ,ppm): δ7.51(s,2H),7.46(s,1H),7.18(m,3H),7.10(m,2H),6.49(s,1H),6.43(s,1H),4.82( s,2H),4.70(s,1H),3.53(s,6H). 13 C NMR (101MHz, CDCl 3 ,ppm)δ167.3,149.3,146.3,143.6,137.4,128.2,128.1,126.6,110.8,109.3,109.2,51.4,51.1,37.3.ESI-MS calcd for C 20 h 19 NO 5 353.1263,found 354.1337[M+H] + ,376.1150[M+Na] + . After mixing the pale yellow solid (1.76 g, 5 mmol) with 210 mmol of hydrazine hydrate, the mixture was stirred under reflux...
Embodiment 2
[0031] The preparation of embodiment 2 compound Co-FPB
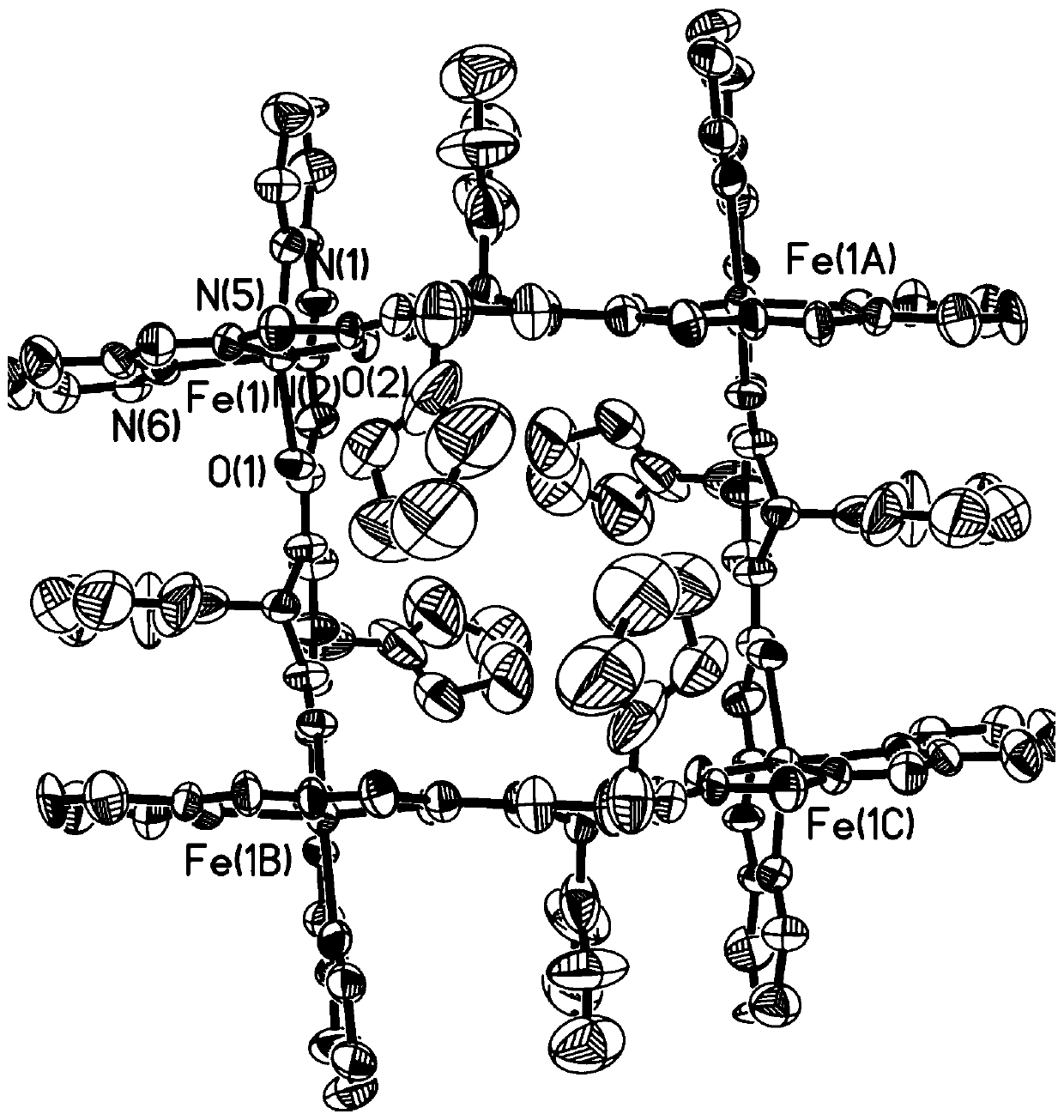
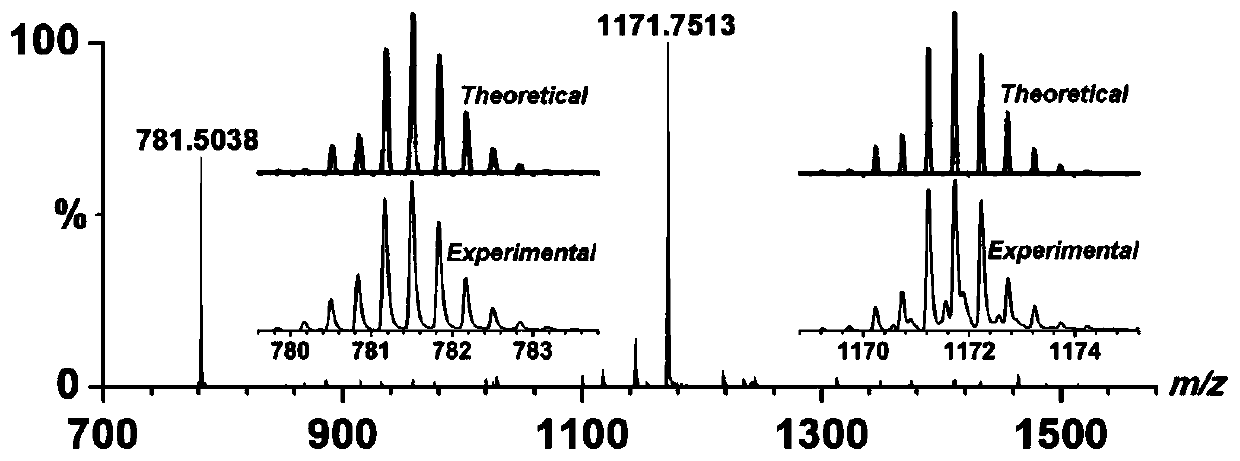
[0032] Co(BF 4 ) 2 ·6H 2 O (34.0mg, 0.10mmol) and the ligand H prepared in Example 1 2 FPB (53.2 mg, 0.10 mmol) was dissolved in 30 ml of dichloromethane and acetonitrile in a mixed solvent with a volume ratio of 1:4, stirred at room temperature for 4 hours, after stirring and filtering, diethyl ether was diffused into the filtrate, and after 2 weeks at room temperature A solid precipitated out of the solution to obtain 27.4 mg of the target compound Co-FPB with a yield of 36%. ESI-MS:m / z:785.4830[H 3 co 4 (FPB) 4 ] 3+ ,1177.6599[H 2 co 4 (FPB) 4 ] 2+ ,1231.2565[H 3 co 4 (FPB) 4 ·BF 4 ] 2+ . The structure crystal diagram of compound Co-FPB, such as image 3 Shown, the solution ESI-MS high-resolution mass spectrum of compound Co-FPB, such as Figure 4 shown.
Embodiment 3
[0033] The preparation of embodiment 3 compound Ni-FPB
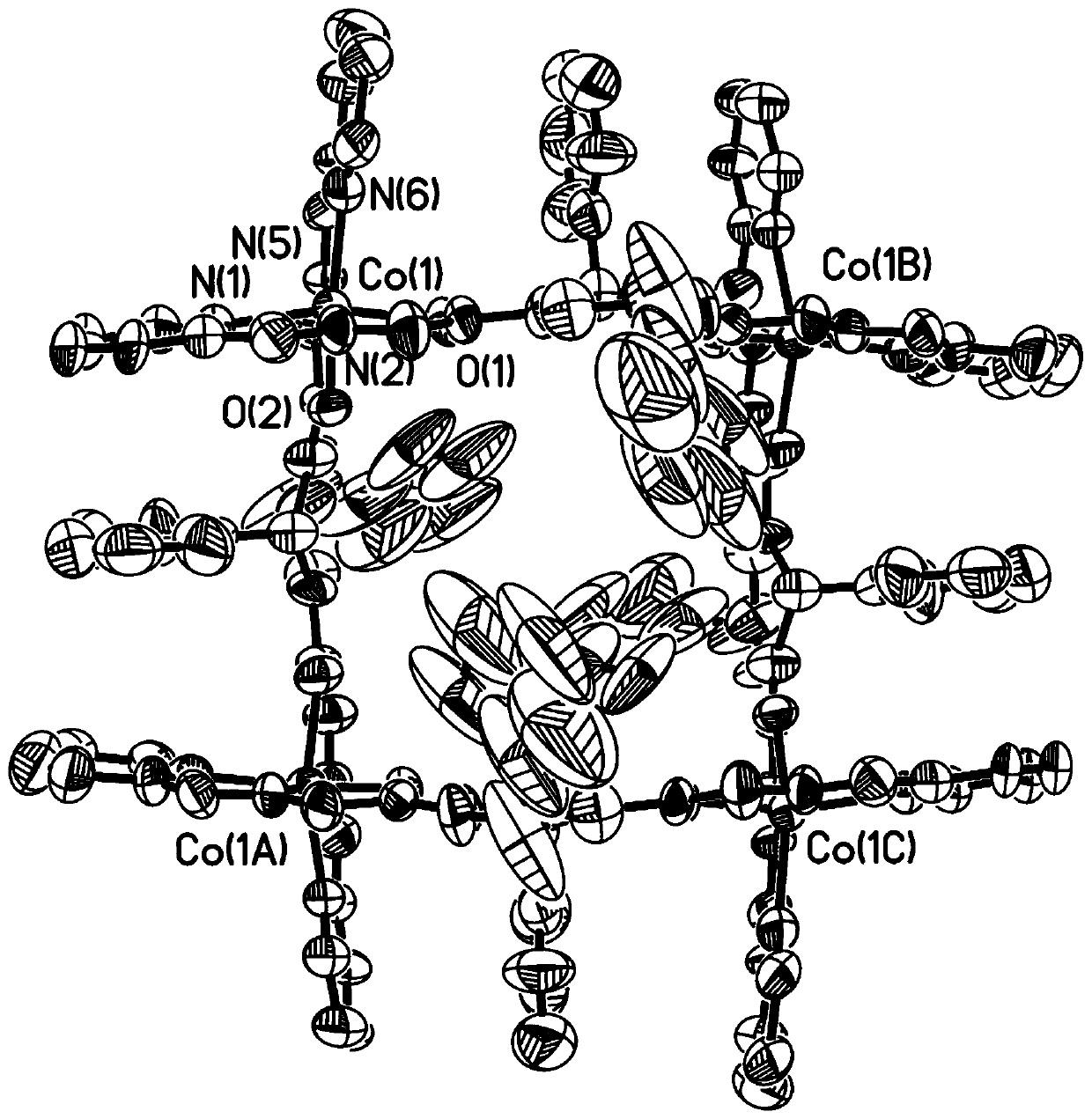
[0034] Ni(ClO 4 ) 2 ·6H 2 O (36.5mg, 0.10mmol) and the ligand H prepared in Example 1 2 FPB (53.2 mg, 0.10 mmol) was dissolved in 30 ml of dichloromethane and acetonitrile in a mixed solvent with a volume ratio of 1:7, stirred at room temperature for 4 hours, after stirring and filtering, diethyl ether was diffused into the filtrate, and after 2 weeks at room temperature A solid precipitated out of the solution to obtain 42.7 mg of the target compound Ni-FPB with a yield of 56%. ESI-MS:m / z:785.1693[H 3 Ni 4 (FPB) 4 ] 3+ ,1177.2492[H 2 Ni 4 (FPB) 4 ] 2+ . The structural crystal diagram of the compound Ni-FPB, such as Figure 5 Shown, the solution ESI-MS high-resolution mass spectrum of compound Ni-FPB, such as Figure 6 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com