Compounding method for 4,4'-diaminodiphenylmethane
A technique for the synthesis of diaminodiphenylmethane, which is applied in the preparation of amino compounds, chemical instruments and methods, and the preparation of organic compounds, and can solve the problems of high industrial production costs, limited application range, harsh reactions, etc. Short, simple process, safe production effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
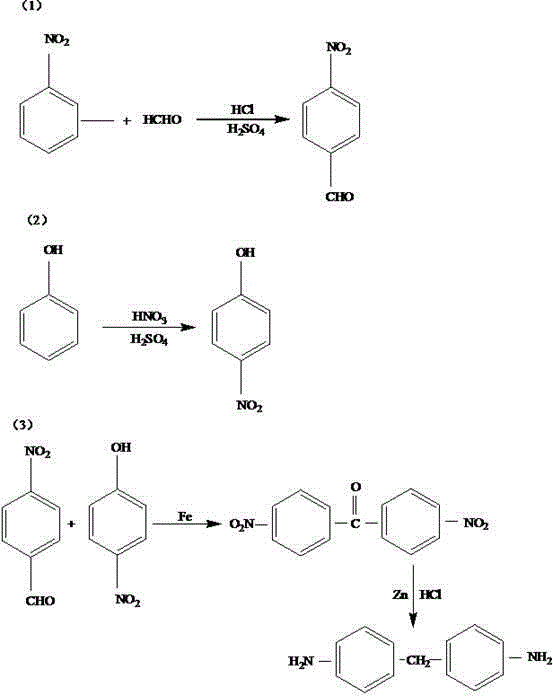
[0022] First, put 150g of nitrobenzene into the reaction kettle, add 200mL of formaldehyde solution with a mass fraction of 45% to it, heat up to 85°C with sealing, and the pressure rises to 1.2MPa. After stirring and reacting for 30min, slowly add 80mL of % hydrogen chloride solution and 30mL sulfuric acid solution with a mass fraction of 70%, keep the temperature constant, continue to stir for 30min, cool down to 25°C, and obtain p-nitrobenzaldehyde after filtration; then take 100g of phenol and put it into the , condensing tube, dropping funnel and thermometer in the four-necked flask, slowly drop 50mL of HNO with a mass concentration of 62% in the form of dropwise addition 3 solution and 30 mL of 70% H 2 SO 4 The solution was added dropwise within 30 minutes, then stirred evenly, heated to 105°C in a water bath, sealed with nitrogen, and the air in the container was discharged. After 15 minutes of reaction, the four-neck bottle was moved to an ice bath. Lower the tempera...
example 2
[0025] First, put 180g of nitrobenzene into the reaction kettle, add 230mL of formaldehyde solution with a mass fraction of 45% to it, seal the temperature to 90°C, and raise the pressure to 1.3MPa. After stirring for 40min, slowly add 83mL of a 35% formaldehyde solution % hydrogen chloride solution and 35mL sulfuric acid solution with a mass fraction of 70%, keep the temperature constant, continue to stir for 40min, cool down to 28°C, and obtain p-nitrobenzaldehyde after filtration; then take 130g phenol and put it into a , condensing tube, dropping funnel and thermometer, slowly drop 70mL of HNO with a mass concentration of 62% in the form of dropwise addition 3 solution and 35 mL of 70% H 2 SO 4The solution was added dropwise within 35 minutes, then stirred evenly, heated to 110°C in a water bath, sealed with nitrogen, and the air in the container was discharged. After 18 minutes of reaction, the four-necked bottle was moved to an ice bath. Lower the temperature to 7°C an...
example 3
[0028] First, put 200g of nitrobenzene into the reaction kettle, add 250mL of formaldehyde solution with a mass fraction of 45% to it, seal the temperature to 100°C, and raise the pressure to 1.5MPa. After stirring for 50min, slowly add 85mL of a 35% formaldehyde solution. % hydrogen chloride solution and 40mL sulfuric acid solution with a mass fraction of 70%, keep the temperature constant, continue to stir for 50min, cool down to 30°C, and obtain p-nitrobenzaldehyde after filtration; then take 150g of phenol and put it into the , condensing tube, dropping funnel and thermometer, slowly drop 80mL of HNO with a mass concentration of 62% in the form of dropwise addition 3 solution and 40 mL of 70% H 2 SO 4 The solution was added dropwise within 40 minutes, then stirred evenly, heated to 115°C in a water bath, sealed with nitrogen, and the air in the container was exhausted. After 20 minutes of reaction, the four-necked bottle was moved to an ice bath. Lower the temperature to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com