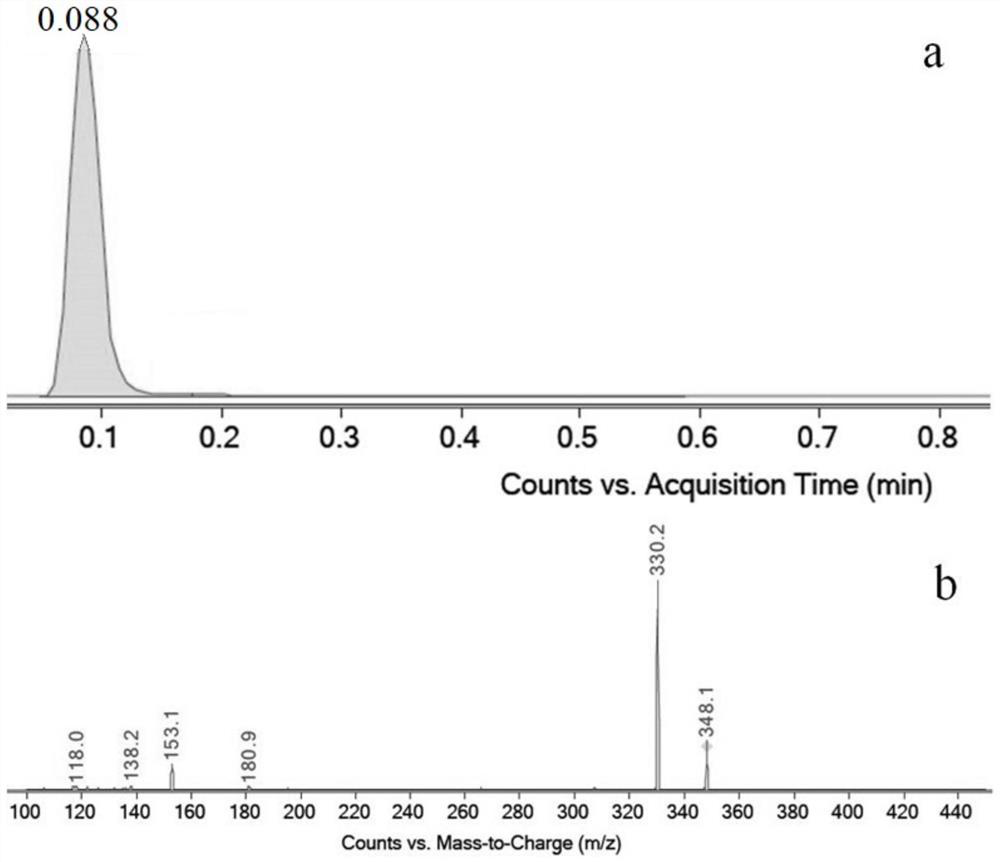
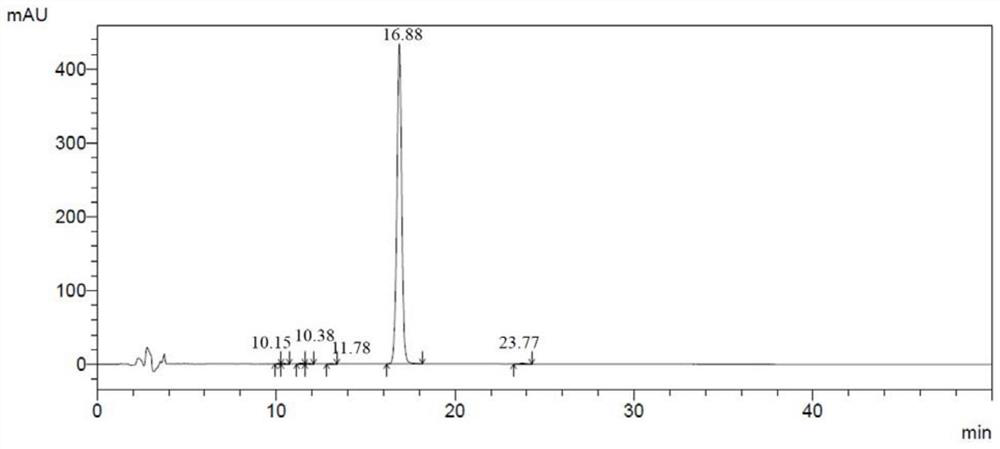
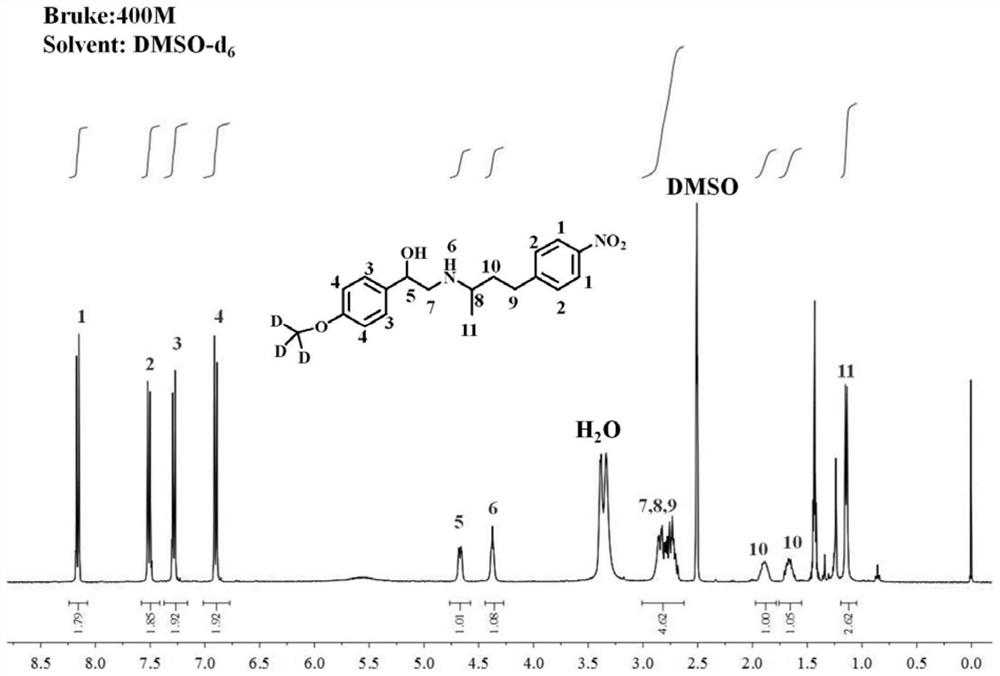
Synthetic preparation method of stable isotope labeled phenylethanolamine A
A stable isotope and phenylethanolamine technology, which is applied in the preparation of carbon-based compounds, organic compounds, and aminohydroxyl compounds, can solve the problems of high price and high detection cost, and achieve fewer experimental steps and lower detection costs , The effect of low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The synthetic preparation method of the stable isotope-labeled phenylethanolamine A provided by the present invention comprises the following steps:
[0028] S1) p-Nitrobenzaldehyde is first combined with acetyltriphenylphosphine, and then reduced by trichlorosilane to obtain the compound shown in formula 1;
[0029]
[0030] S2) the compound of formula 1 undergoes reductive amination reaction to obtain the compound shown in formula 2;
[0031]
[0032] S3) bromo-p-hydroxyacetophenone and deuterated methanol are reacted by Mitsunobu to obtain deuterium-labeled bromo-p-methoxyacetophenone shown in formula 3;
[0033]
[0034] S4) The compounds of formula 2 and formula 3 are subjected to a nucleophilic reaction in methanol, and directly reduced with a hydroboration reagent to obtain the stable isotope-labeled phenylethanolamine A of the target compound, the structural formula is as follows:
[0035]
[0036] The synthetic route of the stable isotope-labeled p...
Embodiment 1
[0040] (1) Dissolve 6.04g of p-nitrobenzaldehyde and 12.75g of acetyltriphenylphosphine in 60mL of dichloromethane, react at 50°C for 12 hours, then cool to room temperature, add 8mL of trichlorosilane to the solution, and react at room temperature for 24 hours . At the end, add 120 mL of saturated NaHCO 3 , after stirring for 2 hours, suction filtration. Then the aqueous phase was extracted with ethyl acetate, a total of 2 extractions, 60 mL each time, the organic phases were combined, dried with anhydrous sodium sulfate, filtered with suction, the solvent was removed by rotary evaporation, and purified by chromatography. The eluent used was a mixture of n-hexane and ethyl acetate at a molar ratio of 4:1 to obtain 6.64 g of the compound of formula 1 with a yield of 86.7%.
[0041] (2) After dissolving 2.15 g of the compound of formula 1 in 28 mL of 2M ethanol-ammonia solution, add 6.67 mL of isopropyl titanate and react at room temperature for 6 hours. Subsequently, the re...
Embodiment 2
[0045] (1) Dissolve 12g of p-nitrobenzaldehyde and 25.5 g of acetyltriphenylphosphine in 120 mL of dichloromethane, react at 70°C for 6 hours, then add 16 mL of trichlorosilane to the solution, and finish the reaction after 6 hours. Then add 300 mL saturated NaHCO 3 , after stirring for 2 hours, the aftertreatment was the same as step 1 in Example 1 to obtain 10.5 g of the compound of formula 1 with a yield of 68.4%.
[0046] (2) After 10.5 g of the compound of formula 1 was dissolved in 150 mL of 2M methanol-ammonia solution, 35 mL of isopropyl titanate was added and reacted under ice-cooling for 12 hours. Subsequently, 3.5 g of sodium borohydride was added, and after the addition was completed, the reaction was returned to room temperature for 3 hours. Subsequently, 50 mL of saturated sodium carbonate solution was added, and after stirring for 2.5 hours, the aftertreatment was the same as step 2 in Example 1 to obtain 9.46 g of the compound of formula 2 with a yield of 90.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com