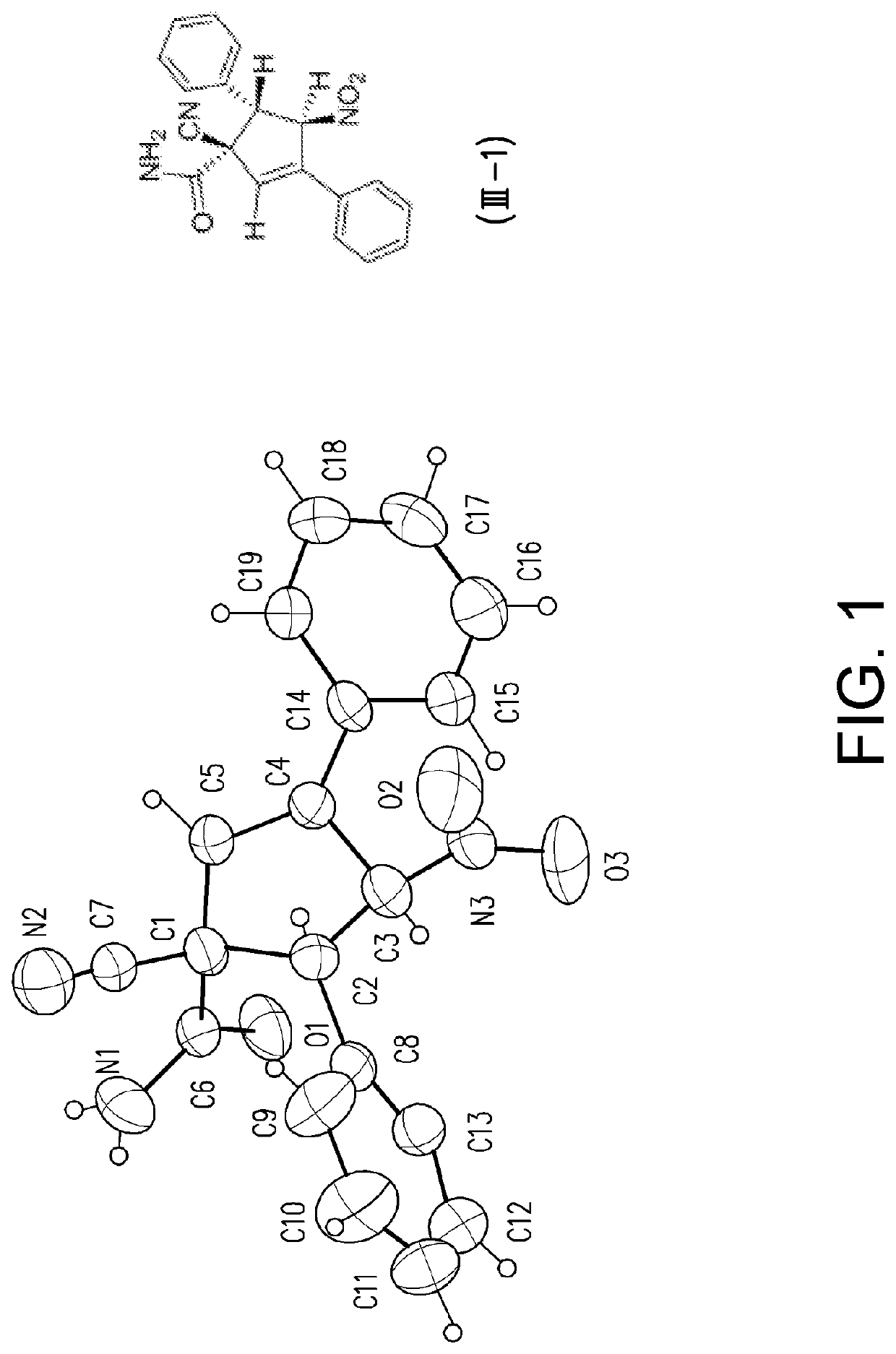
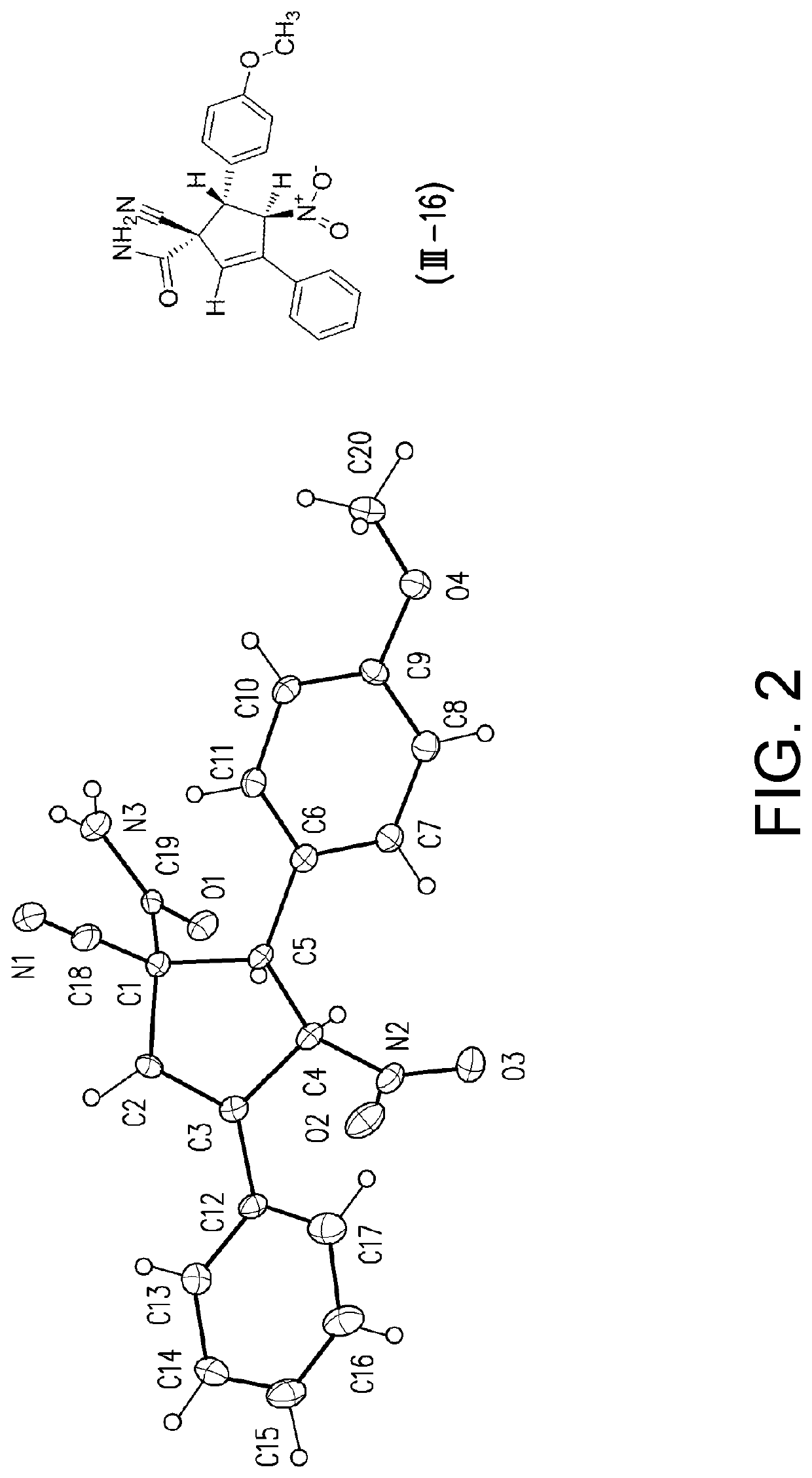
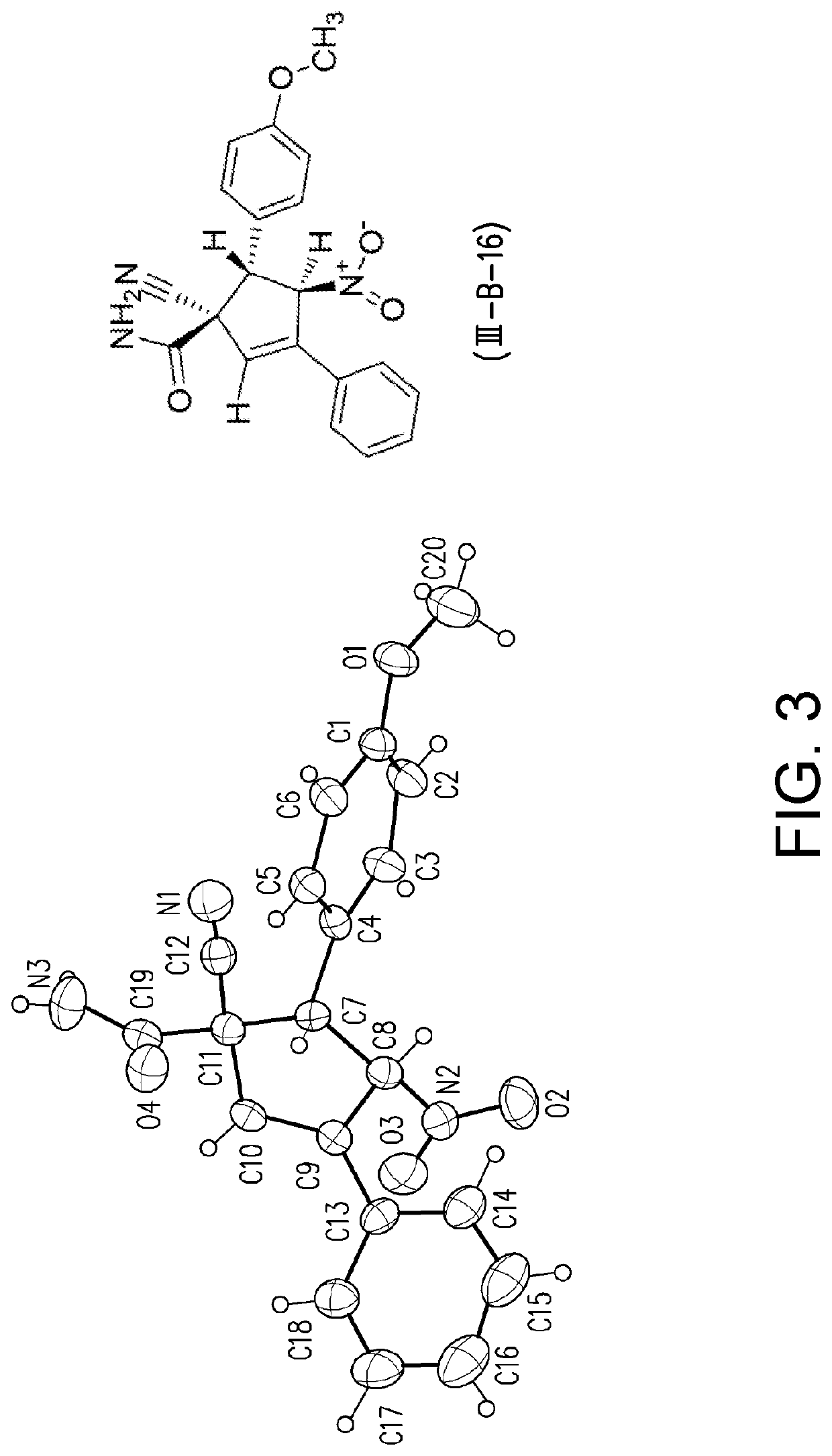
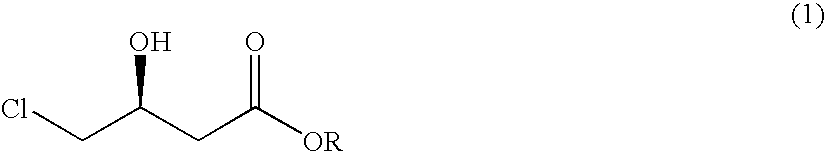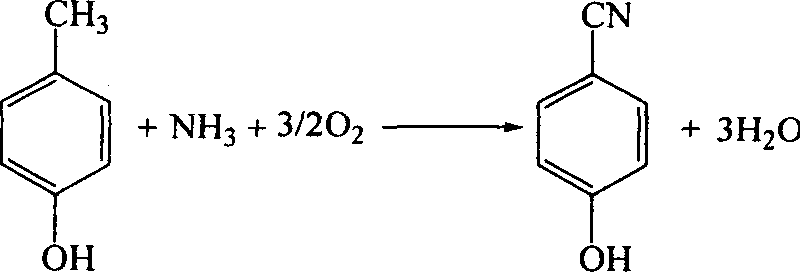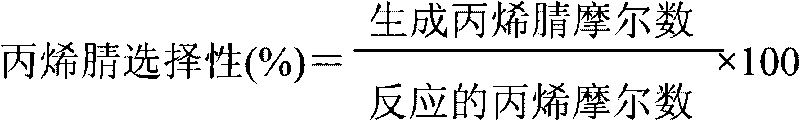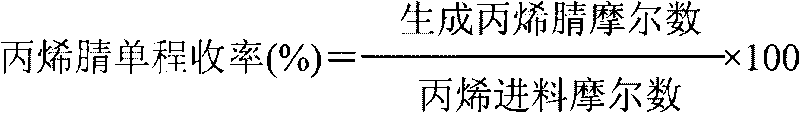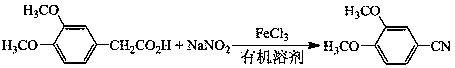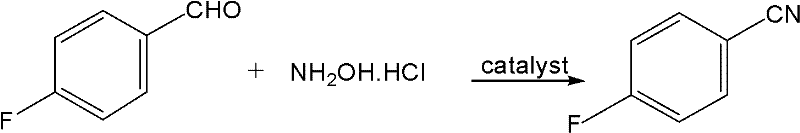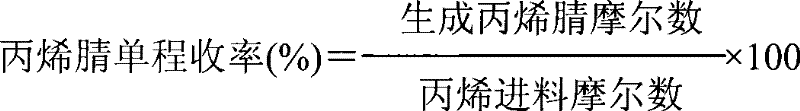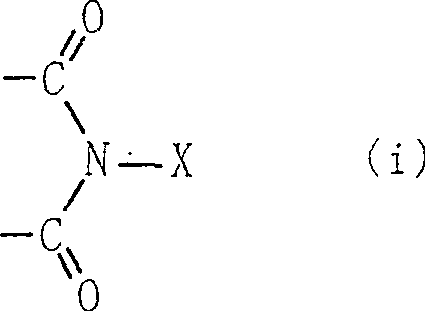Patents
Literature
37results about "Preparation by nitrogen oxide-organic compound reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Process for the preparation of a nickel/phosphorous ligand catalyst for olefin hydrocyanation
ActiveCN1735460AOrganic-compounds/hydrides/coordination-complexes catalystsNickel organic compoundsChlorideSolvent
A process for preparing a hydrocyanation catalyst comprising contacting a bidentate phosphorous-containing ligand with nickel chloride in the presence of a nitrile solvent and a reducing metal which is more electropositive than nickel the nickel chloride being introduced as an aqueous solution and the water being removed concurrently with the reduction reaction by azeotropic distillation.
Owner:INVISTA TECHNOLOG IES S A R L
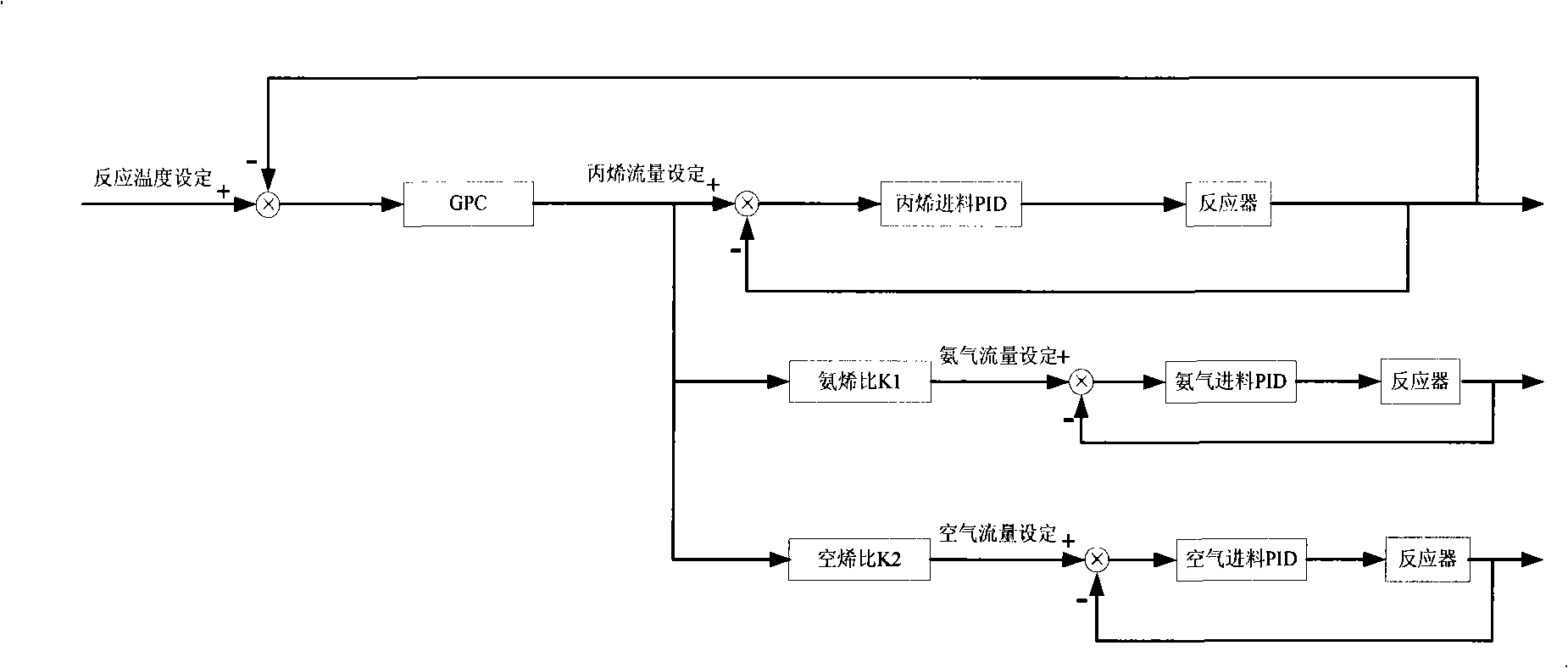
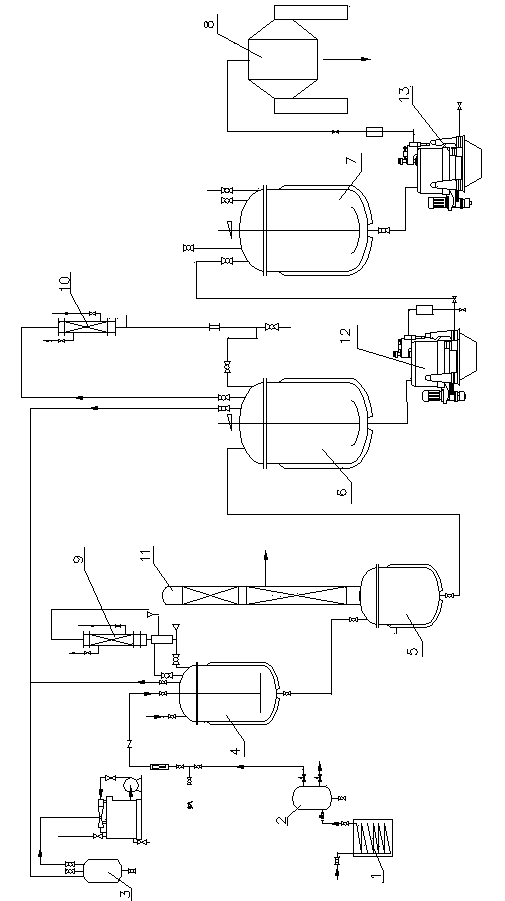
Production device for acrylic nitrile and method for controlling temperature of reactor
InactiveCN101284801ASmooth changeExtended service lifePreparation by nitrogen oxide-organic compound reactionTemperature control using electric meansTemperature controlAcrylonitrile
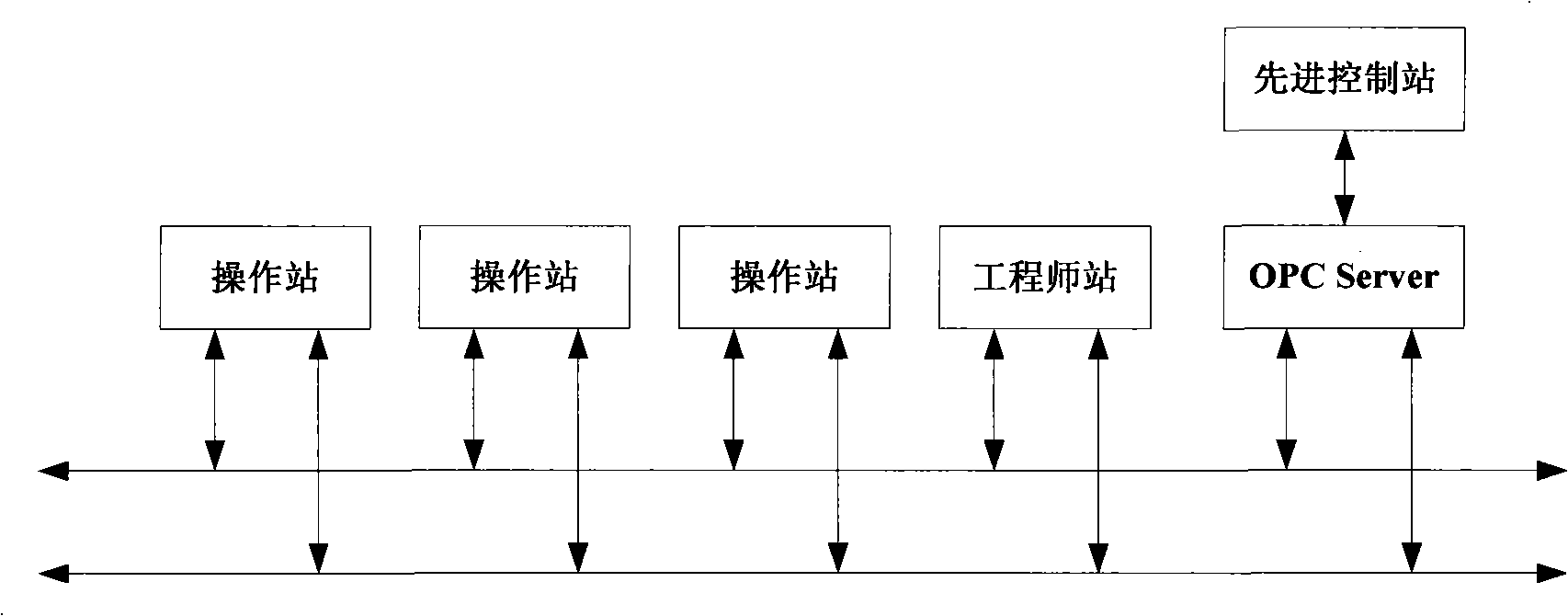
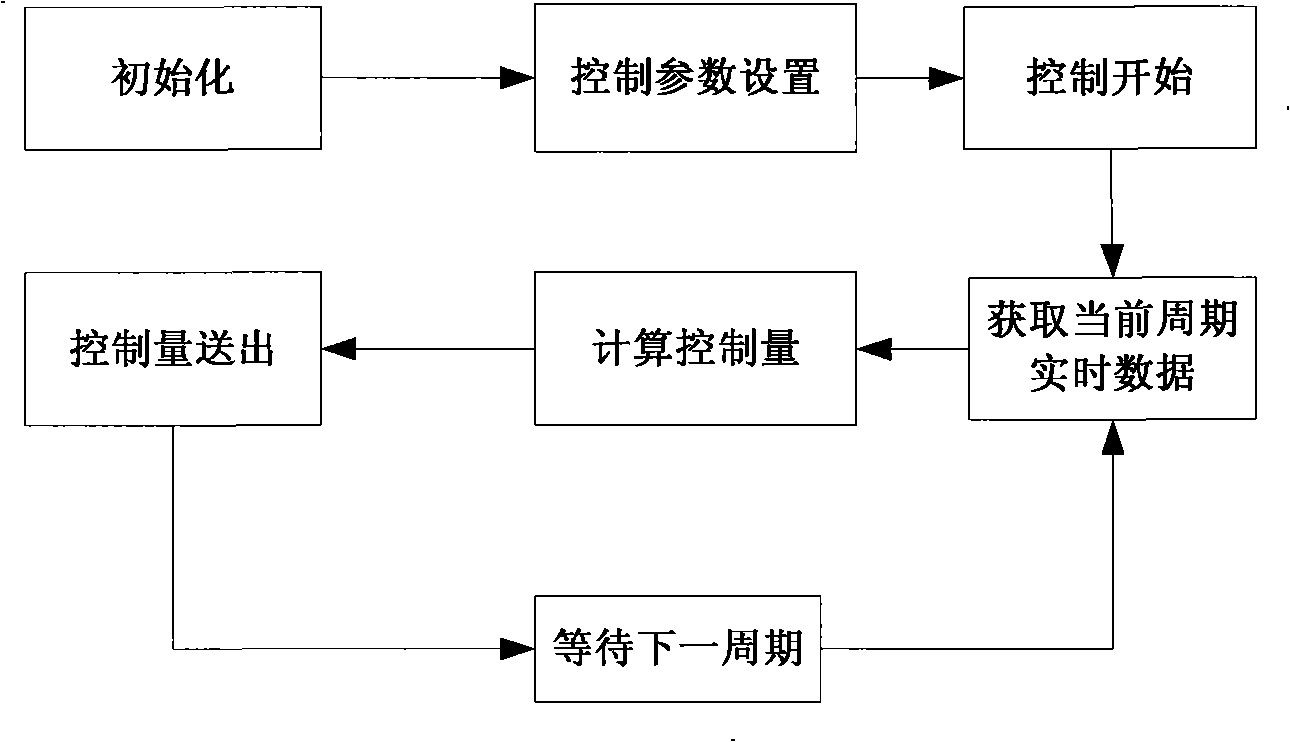
The invention discloses an acrylonitrile production device and a method for controlling the temperature of a reactor thereof, wherein, the acrylonitrile production device comprises a GPC controller and a PID feeding control circuit, and the output end of the GPC controller is connected with the input end of the PID feeding control circuit. When the temperature control is performed, firstly, a GPC control algorithm is adopted to modify a preset value of feeding flow of a reactor, according to the preset temperature information and the actual temperature information of the reactor, to obtain an actual preset value of the feeding flow; then the feeding flow is adjusted through the PID algorithm to realize the control to the temperature of the rector. The method can ensure that the reactor has steady temperature control, steady change of an actuating mechanism, and long service life.
Owner:UNIV OF SCI & TECH OF CHINA
Process for the preparation of a nickel/phosphorous ligand catalyst for olefin hydrocyanation
ActiveCN100348322COrganic-compounds/hydrides/coordination-complexes catalystsNickel organic compoundsChlorideSolvent
A process for preparing a hydrocyanation catalyst comprising contacting a bidentate phosphorous-containing ligand with nickel chloride in the presence of a nitrile solvent and a reducing metal which is more electropositive than nickel the nickel chloride being introduced as an aqueous solution and the water being removed concurrently with the reduction reaction by azeotropic distillation.
Owner:INVISTA TECHNOLOG IES S A R L
Industrialized method for producing 2, 6-dichloro phenyl nitrile
InactiveCN1775747ABiocidePreparation by nitrogen oxide-organic compound reactionTolueneMedicinal chemistry
The invention relates to a method for industrialized producing 2.6-dichlorobenzonitrile, adopting 6-chloro-o-nitrotoluene chloro-o-nitrotoluene as raw material to make chlorination reaction under the action of catalyst, then making cyanogenation reaction with formic acid and hydroxylammonium chloride, and refining again and again to obtain 2.6-dichlorobenzonitrile whose content is 99.8% above. The produced 2.6- dichlorobenzonitrile is a white powdery crystal, purity 99.8%-9995%, melting point 141.0 deg.C -144.0 deg.C, water ratio <=01%, and product yield up to 64%.
Owner:YANGZHOU TIANCHEN FINE CHEM
Preparation method for m-xylylenediamine
ActiveCN109456200AReduce usageReduce energy consumptionOrganic compound preparationPreparation by nitrogen oxide-organic compound reactionCarboxylic acidToluene
The invention provides a preparation method for m-xylylenediamine. In the method, the m-xylylenediamine is converted into isophthalonitrile through ammoxidation; the obtained isophthalonitrile is extracted by using N-alkylpyrazole; after carboxylic acid is removed from extracted isophthalonitrile solution through resin, the isophthalonitrile solution enters a hydrogenation reactor for performing ahydrogenation reaction, to obtain a m-xylylenediamine reaction solution; and finally, the m-xylylenediamine with a high purity (99.9%) is obtained by rectifying and separating. The method has the characteristics of simple reaction flow, low energy consumption, simple operation, high m-xylylenediamine yield, high m-xylylenediamine purity and the like.
Owner:WANHUA CHEM GRP CO LTD
Method for preparing 2, 6-dichlorobenzonitrile
ActiveCN103382166AReduce consumptionReduce generationPreparation by nitrogen oxide-organic compound reactionNitrosoChemical synthesis
The invention discloses a method for preparing 2, 6-dichlorobenzonitrile, and relates to the technical field of production of chemically synthesized 2, 6-dichlorobenzonitrile. According to the method, 2, 6-dichlorotoluene is used as a starting material and chlorinated to prepare 2, 6-benzylidene chloride, the 2, 6-benzylidene chloride is hydrolyzed and quaternized to prepare a 2, 6-dichlorobenzonitrile crude product, and the 2, 6-dichlorobenzonitrile crude product is refined to obtain a 2, 6-dichlorobenzonitrile fine product. Production is easily controlled, raw material consumption is less, production cost is low, solid waste is decreased, emission of nitrogenous nitroso-group waste gas is reduced, and the method is an energy-saving, emission-reduction and environment-friendly industrial practical cleaning production technique.
Owner:永椿化工新材料有限公司
Process for preparing 4-chloro-3-hydroxybutanoic acid ester
InactiveUS20060264652A1Improve productivityDecrease in reaction stepOrganic compound preparationOrganic chemistry methodsCyanideAlcohol
The present invention relates to a process for preparing 4-chloro-3-hydroxybutanoic acid ester, an intermediate for preparing atorvastatin, in high purity and yield, by comprising the steps of 1) reacting epichlorohydrin of formula (2) with cyanide of formula (3) under the condition of pH ranging from 7 to 8, to form the 4-chloro-3-hydroxybutyronitrile of formula (4) and 2a) dissolving the 4-chloro-3-hydroxybutyronitrile of formula (4) in an alcoholic solvent and reacting it with hydrogen chloride, or 2b) reacting the 4-chloro-3-hydroxybutyronitrile of formula (4) in an alcoholic solvent saturated with hydrogen chloride, to form the 4-chloro-3-hydroxybutyronitrile acid ester of formula (I).
Owner:LG LIFE SCI
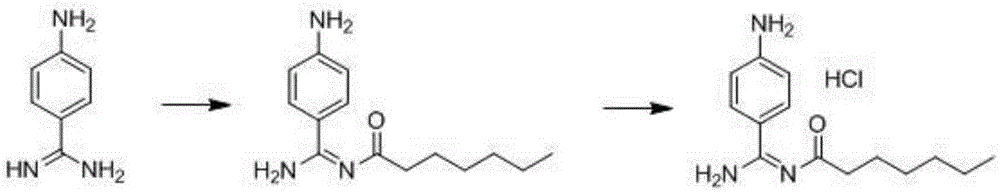
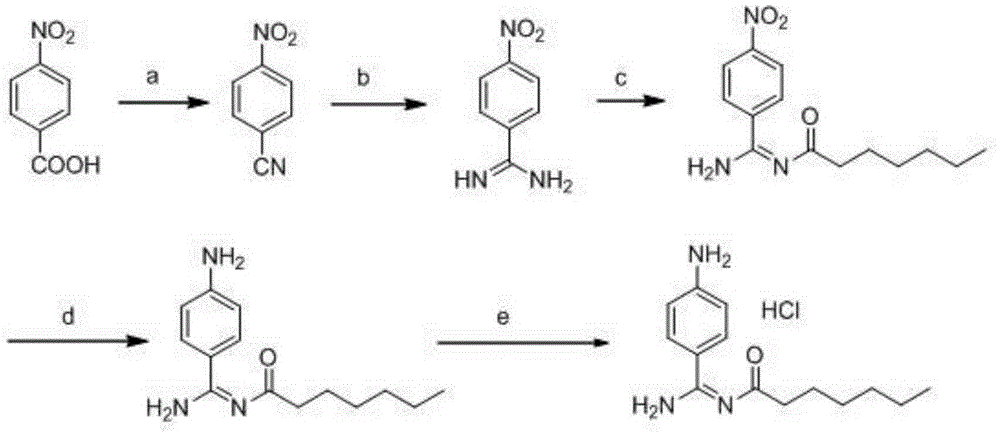
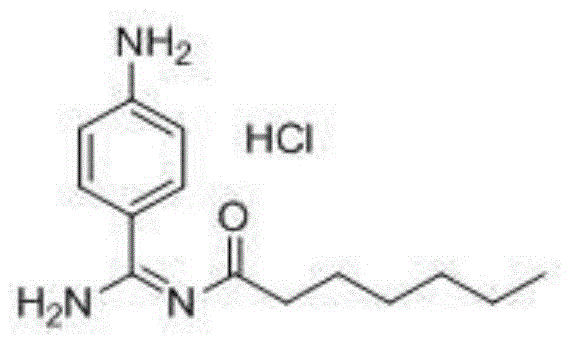
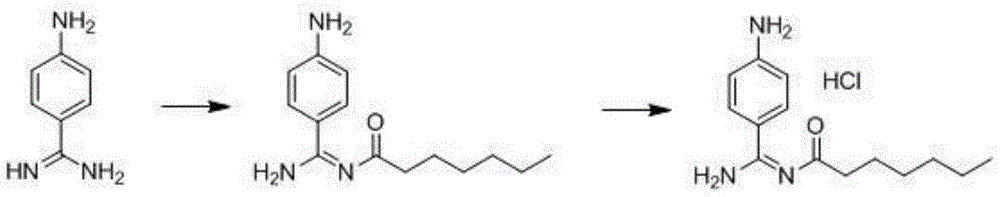
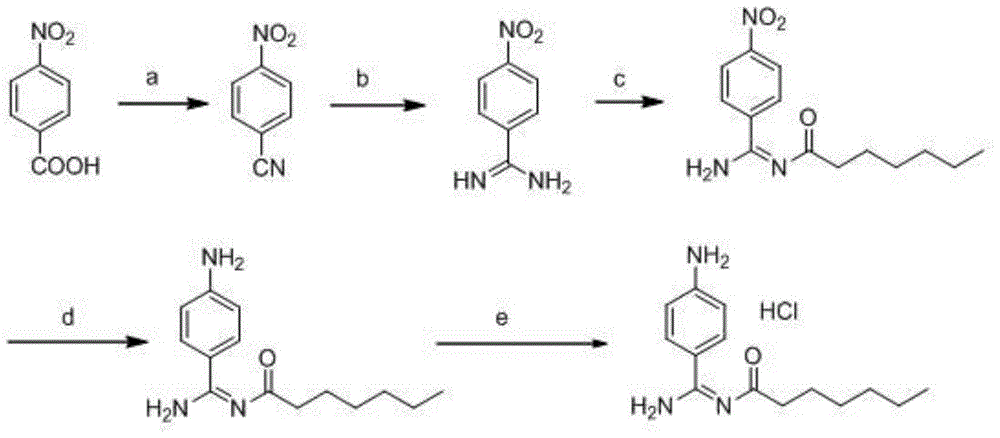
Preparation method for p-aminobenzamidine hydrochloride
InactiveCN105330568AEasy to operateEasy to controlPreparation by nitrogen oxide-organic compound reactionP-aminobenzamidineDrugs synthesis
The invention discloses a preparation method for p-aminobenzamidine hydrochloride, and belongs to the field of drug synthesis. The preparation method comprises the following steps: taking p-aminobenzamidine as a starting raw material, and enabling the p-aminobenzamidine and hydroxylamine hydrochloride to generate nitrobenzonitrile; then, enabling the nitrobenzonitrile to react with ammonium salt to generate p-aminobenzamidine; carrying out acylation and reduction on the p-aminobenzamidine to obtain p-aminobenzamidine imidogen n-hexyl formate; finally, performing salifying on the p-aminobenzamidine imidogen n-hexyl formate and hydrogen chloride to obtain the p-aminobenzamidine hydrochloride. Compared with the prior art, the preparation method for the p-aminobenzamidine hydrochloride disclosed by the invention has the characteristics of being simple to operate, easy to control in reaction, low in production cost and the like, and has a very good popularization and application value.
Owner:INST OF PHARMACY SHANDONG PROV ACAD OF MEDICAL SCI
Catalyst for synthesizing para-hydroxy-benzonitrile and its preparing method and use
InactiveCN1820847ALow molar ratioHigh selectivityCatalyst carriersCatalyst activation/preparationBenzonitrileAmmonia
The catalyst for synthesizing para-hydroxy-benzonitrile includes main catalyst and co-catalyst. The main catalyst has active matters MoO3, Cr2O3 and P2O5 in the atom ratio of Mo:Cr:P=1:1.3-10:15-75; and the co-catalyst has active matter of at least one of the oxide of Fe, Ti, Bi, Sb, Li, Na, K, Rb and Cs, and the atom ratio to Mo of 0.1-11. Using the catalyst of the present invention in ammoxidation process to synthesie para-hydroxy-benzonitrile has high product selectivity and yield, long catalyst service life, small ammonia ratio and excellent application foreground.
Owner:WUHAN UNIV
Production device for preparing 2, 6-dichlorobenzonitrile
ActiveCN103382165AReduce consumptionReasonable designPreparation by nitrogen oxide-organic compound reactionEnvironmental resistanceBuffer tank
The invention discloses a production device for preparing 2, 6-dichlorobenzonitrile, and relates to the technical field of production of chemically synthesized 2, 6-dichlorobenzonitrile. The production device comprises an evaporator, a buffer tank, a hydrogen chloride recycling tank, a chlorination kettle, a first rectifying still and a hydrolysis quaternizing kettle. The production device is reasonable in design, convenient to control, less in raw material consumption and low in production cost, solid waste is decreased, emission of nitrogenous nitroso-group waste gas is reduced, and the production device is an energy-saving, emission-reduction and environment-friendly industrial practical cleaning production technique.
Owner:永椿化工新材料有限公司
High-stability fluid catalyst for producing acrylonitrile
ActiveCN101733117AHigh purityPromote conversionPreparation by nitrogen oxide-organic compound reactionMetal/metal-oxides/metal-hydroxide catalystsSilicon dioxideAmmoxidation
The invention relates to a high-stability fluid catalyst for producing acrylonitrile by propylene ammoxidation, which mainly solves the problem of poor stability of catalysts in the prior art. The high-stability fluid catalyst adopts silicon dioxide, aluminum oxide or a mixture thereof as a carrier and comprises a composition with the following chemical formula in atomic ratio: AaBbGecBafPrdBieMo12Ox, wherein A is at least one of Li, Na, K, Rb or Cs; and B is at least one of Ca, Mn, Fe, Co or Ni. The technical scheme solves the problem better, and can be used for industrial production of the acrylonitrile under the high propylene loading condition.
Owner:CHINA PETROLEUM & CHEM CORP +1
Preparation method of 3, 4-dimethoxybenzonitrile
ActiveCN110668972AImprove securitySimple stepsPreparation by nitrogen oxide-organic compound reactionNitric oxidePtru catalystNitrogen source
A preparation method of 3,4-dimethoxybenzonitrile is characterized in that 3,4-dimethoxyphenylacetic acid and sodium nitrite are used as raw materials, and ferric trichloride is used as a catalyst. The preparation method which is different from previous synthesis methods has the following characteristics: the used raw materials are cheap and easily available phenylacetic acid compounds, and the sodium nitrite is used as a nitrogen source, so the toxicity of the raw materials is low, and the price is low. The problem of high comprehensive cost of materials such as reaction reagents used in theexisting method is solved. The preparation method of the 3,4-dimethoxybenzonitrile has the advantages of low toxicity, simple process, mild conditions and high finished product yield.
Owner:河南睿嵩检测技术有限公司
One-pot aromatic nitrile synthesis method adopting Fe (III) porphyrin for catalyzing nitrite to oxidize aromatic olefin
InactiveCN107602415AMild reaction conditionsImprove responseOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAir atmosphereSynthesis methods
The invention discloses a one-pot aromatic nitrile synthesis method adopting Fe (III) porphyrin for catalyzing nitrite to oxidize aromatic olefin. According to the method, in an air atmosphere and anorganic carboxylic acid solution system, aromatic nitrile compounds or aromatic heterocyclic nitrile compounds are generated from aromatic alkene compounds or aromatic heterocyclic alkene compounds and nitrite under the catalysis of Fe (III) porphyrin by one-step reaction. The method has the advantages as follows: 1) reaction conditions are mild, operation is simple and easy to control, and yieldis higher; 2) an efficient Fe (III) porphyrin catalyst instead of a toxic CN negative ion reagent is adopted, so that environmental pollution is reduced; 3) raw materials, the nitrogen source, an acidreagent and the like are cheap and easily available, the production cost is obviously reduced, and the method can be popularized and applied to industrial production.
Owner:YUANJIANG HUALONG CATALYST TECH
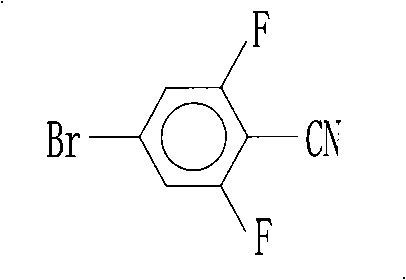
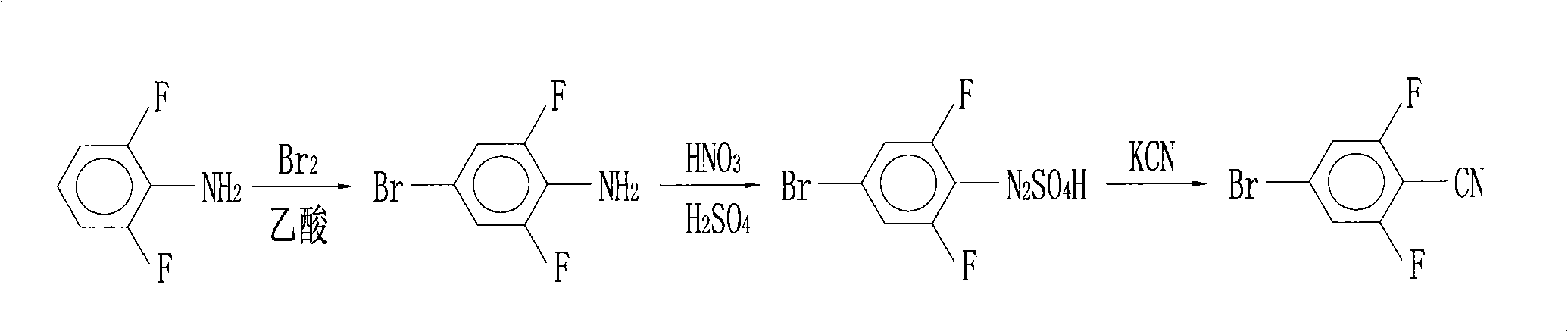
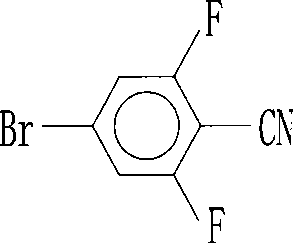
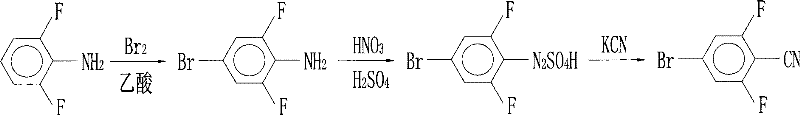
Preparation of 4-bromo-2,6-difluorobenzonitrile
InactiveCN101353317AReduce pollutionLow pricePreparation by nitrogen oxide-organic compound reactionPotassium cyanideFiltration
The invention discloses a preparation method of 4-bromo-2, 6-difluorobenzonitrile. In the preparation method, tetrahydrofuran, potassium tert butoxide, petroleum ether, 3, 5-difluorobromobenzene, DMF and n-butyllithium with the proportion of n-butyllithium: 3, 5-difluorobromobenzene, which is equal to 1.2:1 and the proportion of DMF: 3, 5-difluorobromobenzene, which is equal to1.6:1 are taken as raw materials to produce 4-bromo-2, 6-difluorobenzaldehyde, and methanoic acid, 4-bromo-2, 6-difluorobenzaldehyde and hydroxylamine hydrochloride with the weight ratio of the methanoic acid, the 4-bromo-2, 6-difluorobenzaldehyde and the hydroxylamine hydrochloride equal to 4:1:1.4 are taken as raw materials to prepare the 4-bromo-2, 6-difluorobenzonitrile. The preparation steps of the 4-bromo-2, 6-difluorobenzonitrile are as follows: A: the raw materials are added to a three-necked flask according to the proportion, and are heated and refluxed for 10 hours at normal pressure; B: a solvent is distilled at normal pressure, then corresponding volume of water is added to the system to continuously distill, and the 4-bromo-2, 6-difluorobenzonitrile is carried out; and C: the distilled aqueous solution is naturally cooled to the room temperature and is carried out suction filtration; a filter cake is washed with water to be neutral, and the product is obtained. The method for preparing the 4-bromo-2, 6-difluorobenzonitrile of the invention takes the 3, 5-difluorobromobenzene as a basic raw material which is cheap, and the whole process engineering is low in cost, so the 4-bromo-2, 6-difluorobenzonitrile is applicable to mass production. The preparation method does not adopt the hypertoxic potassium cyanide and strong corrosive substances such as sulfuric acid, bromine, and the like, which are adopted in the existing process, thus reducing the environmental pollution.
Owner:SHIJIAZHUANG GUODA INDAL
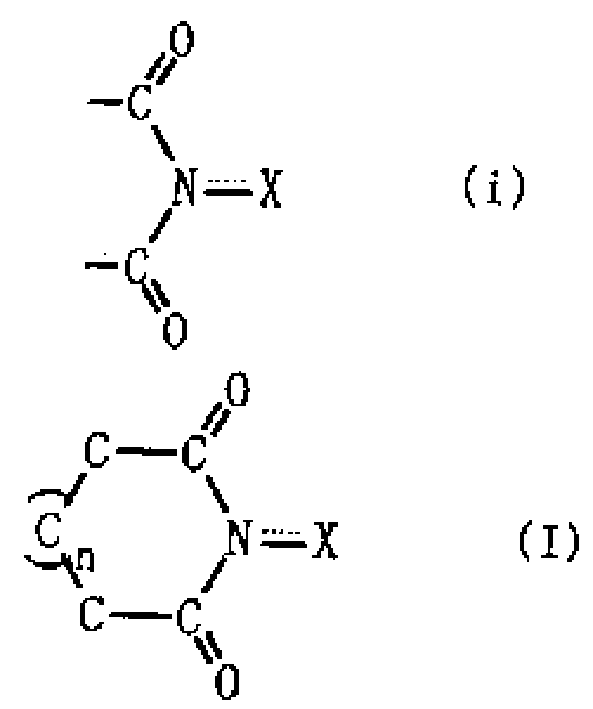
Process for producing organic compound using nitrite
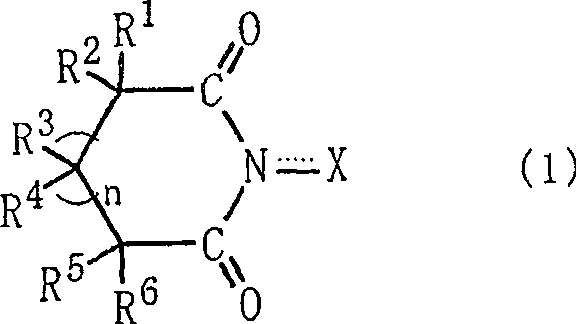
InactiveCN1443734APreparation by oxidation reactionsOrganic compound preparationSimple Organic CompoundsNitrite
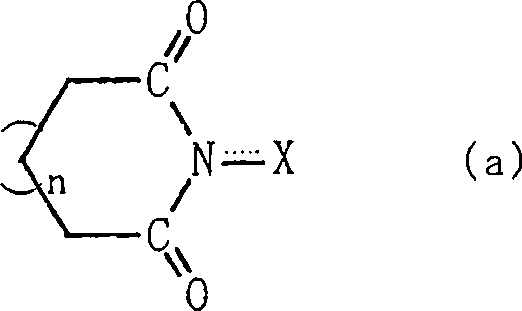
A method for producing an organic compound by making at least one of (A) a compound capable of generating free radicals and (B) an ester and a salt of nitrous acid have, in its ring structure, compositionally represented by the above formula (i) The nitrogen-containing cyclic compound of the skeleton is reacted in the presence of the ring compound, wherein X is one of an oxygen atom and an -OR group, and wherein R is one of a hydrogen atom and a hydroxyl protection group. Examples of nitrogen-containing cyclic compounds include cyclic imide compounds having a cyclic imide skeleton represented by the above formula (I), wherein n is one of 0 and 1; X is one of an oxygen atom and an -OR group species, and wherein R is one of a hydrogen atom and a hydroxyl protecting group.
Owner:DAICEL CHEM IND LTD
Method for preparing 2, 6-dichlorobenzonitrile
ActiveCN103382166BReduce consumptionReduce generationPreparation by nitrogen oxide-organic compound reactionNitrosoChemical synthesis
The invention discloses a method for preparing 2, 6-dichlorobenzonitrile, and relates to the technical field of production of chemically synthesized 2, 6-dichlorobenzonitrile. According to the method, 2, 6-dichlorotoluene is used as a starting material and chlorinated to prepare 2, 6-benzylidene chloride, the 2, 6-benzylidene chloride is hydrolyzed and quaternized to prepare a 2, 6-dichlorobenzonitrile crude product, and the 2, 6-dichlorobenzonitrile crude product is refined to obtain a 2, 6-dichlorobenzonitrile fine product. Production is easily controlled, raw material consumption is less, production cost is low, solid waste is decreased, emission of nitrogenous nitroso-group waste gas is reduced, and the method is an energy-saving, emission-reduction and environment-friendly industrial practical cleaning production technique.
Owner:永椿化工新材料有限公司
Preparation of 4-bromo-2,6-difluorobenzonitrile
InactiveCN101353317BReduce pollutionLow pricePreparation by nitrogen oxide-organic compound reactionFiltrationPotassium cyanide
The invention discloses a preparation method of 4-bromo-2, 6-difluorobenzonitrile. In the preparation method, tetrahydrofuran, potassium tert butoxide, petroleum ether, 3, 5-difluorobromobenzene, DMF and n-butyllithium with the proportion of n-butyllithium: 3, 5-difluorobromobenzene, which is equal to 1.2:1 and the proportion of DMF: 3, 5-difluorobromobenzene, which is equal to1.6:1 are taken as raw materials to produce 4-bromo-2, 6-difluorobenzaldehyde, and methanoic acid, 4-bromo-2, 6-difluorobenzaldehyde and hydroxylamine hydrochloride with the weight ratio of the methanoic acid, the 4-bromo-2, 6-difluorobenzaldehyde and the hydroxylamine hydrochloride equal to 4:1:1.4 are taken as raw materials to prepare the 4-bromo-2, 6-difluorobenzonitrile. The preparation steps of the 4-bromo-2, 6-difluorobenzonitrile are as follows: A: the raw materials are added to a three-necked flask according to the proportion, and are heated and refluxed for 10 hours at normal pressure; B: a solvent is distilled at normal pressure, then corresponding volume of water is added to the system to continuously distill, and the 4-bromo-2, 6-difluorobenzonitrile is carried out; and C: the distilled aqueous solution is naturally cooled to the room temperature and is carried out suction filtration; a filter cake is washed with water to be neutral, and the product is obtained. The method for preparing the 4-bromo-2, 6-difluorobenzonitrile of the invention takes the 3, 5-difluorobromobenzene as a basic raw material which is cheap, and the whole process engineering is low in cost, so the 4-bromo-2, 6-difluorobenzonitrile is applicable to mass production. The preparation method does not adopt the hypertoxic potassium cyanide and strong corrosive substances such as sulfuric acid, bromine, and the like, which are adopted in the existing process, thus reducing the environmental pollution.
Owner:SHIJIAZHUANG GUODA INDAL
A kind of preparation method of m-xylylenediamine
ActiveCN109456200BAvoid thermal decompositionIncrease profitOrganic compound preparationPreparation by nitrogen oxide-organic compound reactionHydrogenation reactionPhysical chemistry
The invention provides a preparation method of m-xylylenediamine. In this process, m-xylene is converted to isophthalonitrile by ammoxidation. The resulting isophthalonitrile is extracted with N-alkylpyrazoles. The extracted isophthalonitrile solution is decarboxylated by the resin and then enters the hydrogenation reactor for hydrogenation reaction to obtain the m-xylylenediamine reaction liquid. Finally, high-purity (>99.9%) m-xylylenediamine was obtained through rectification and separation. The method has the characteristics of simple reaction process, low energy consumption, simple operation, high yield of m-xylylenediamine, high purity of m-xylylenediamine, and the like.
Owner:WANHUA CHEM GRP CO LTD
Synthetic method of dichlorobenzonitrile
InactiveCN102391152AMild reaction conditionsSimple stepsPreparation by nitrogen oxide-organic compound reactionChemical recyclingOrganic solventCyanide
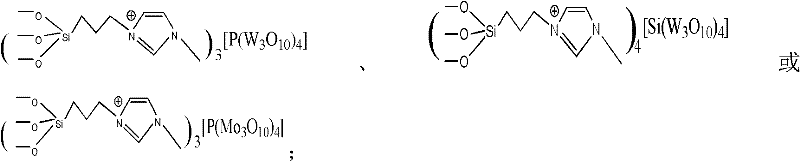
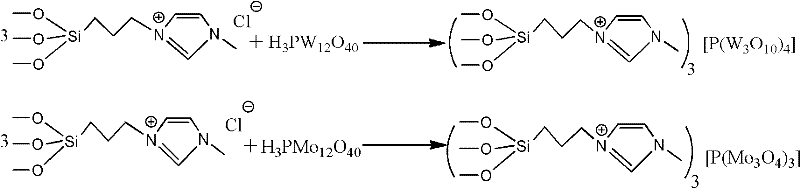
The invention relates to a synthetic method of dichlorobenzonitrile, which is characterized in that dichlorobenzonitrile and oxammonium hydrochloride are resolved in an organic solvent, and the dichlorobenzonitrile is catalyzed and synthesized through a heteropoly salt catalyst, the reaction temperature is controlled to range from 80 to130 DEG C, and the reaction lasts for 8 to 12h. In the invention, cyanide is not used as raw materials, the method is simple and feasible, the product yield is high, the process flow is simple, the requirements of equipment are low, and the catalyst can be recovered for reutilization.
Owner:NANJING UNIV OF TECH
High-stability fluid catalyst for producing acrylonitrile
ActiveCN101733117BPreparation by nitrogen oxide-organic compound reactionMetal/metal-oxides/metal-hydroxide catalystsSilicon dioxideAmmoxidation
The invention relates to a high-stability fluid catalyst for producing acrylonitrile by propylene ammoxidation, which mainly solves the problem of poor stability of catalysts in the prior art. The high-stability fluid catalyst adopts silicon dioxide, aluminum oxide or a mixture thereof as a carrier and comprises a composition with the following chemical formula in atomic ratio: AaBbGecBafPrdBieMo12Ox, wherein A is at least one of Li, Na, K, Rb or Cs; and B is at least one of Ca, Mn, Fe, Co or Ni. The technical scheme solves the problem better, and can be used for industrial production of the acrylonitrile under the high propylene loading condition.
Owner:CHINA PETROLEUM & CHEM CORP +1
Perfuming ingredients of the floral and/or anis type
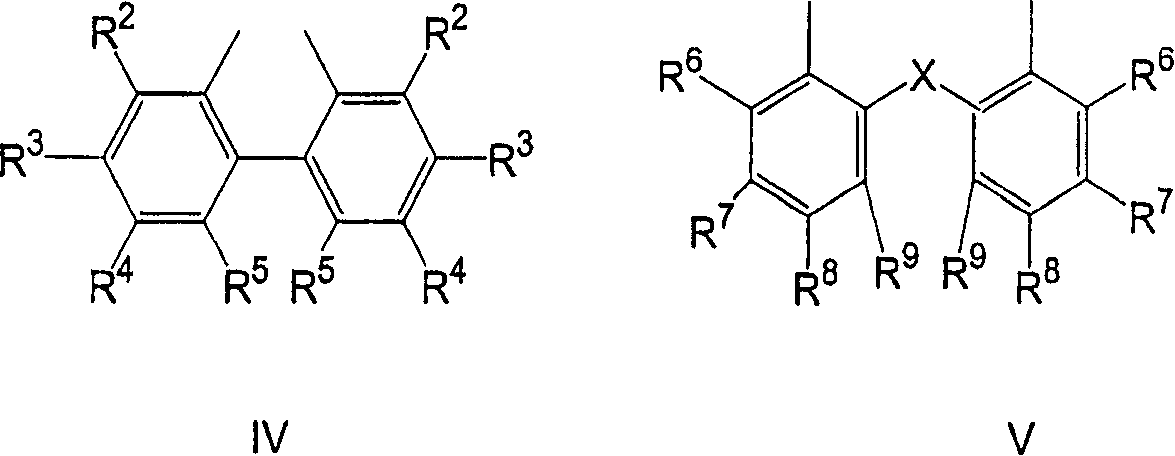
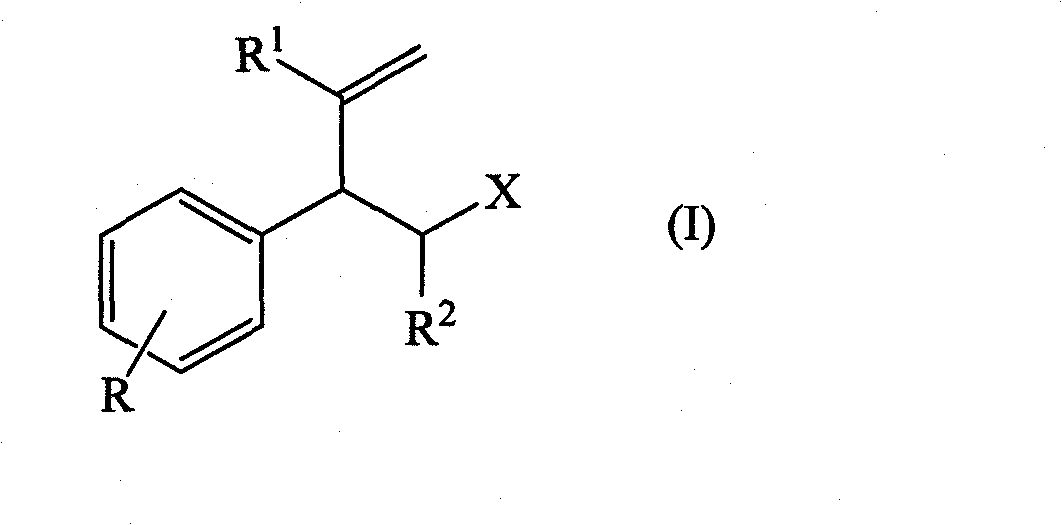
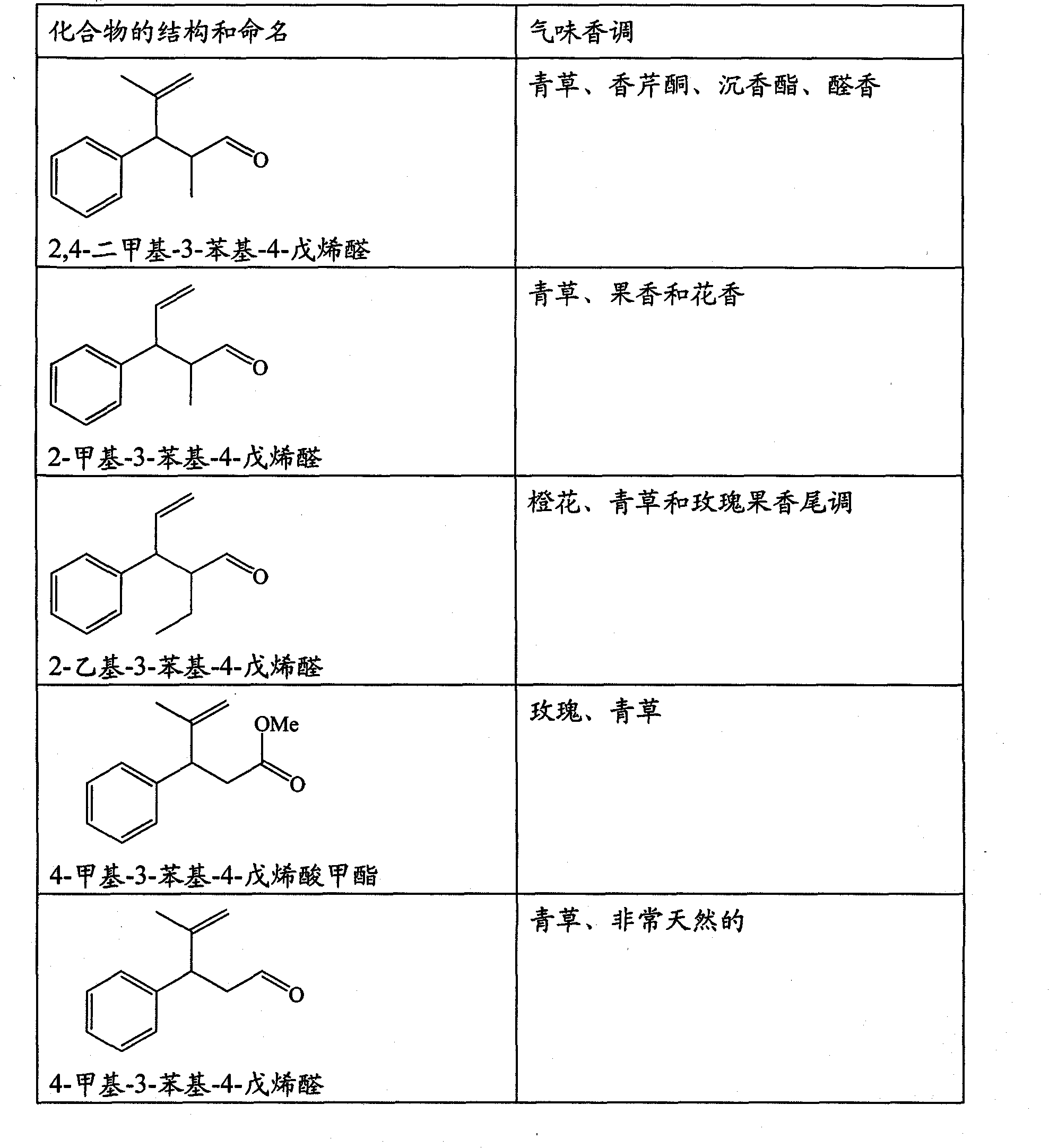
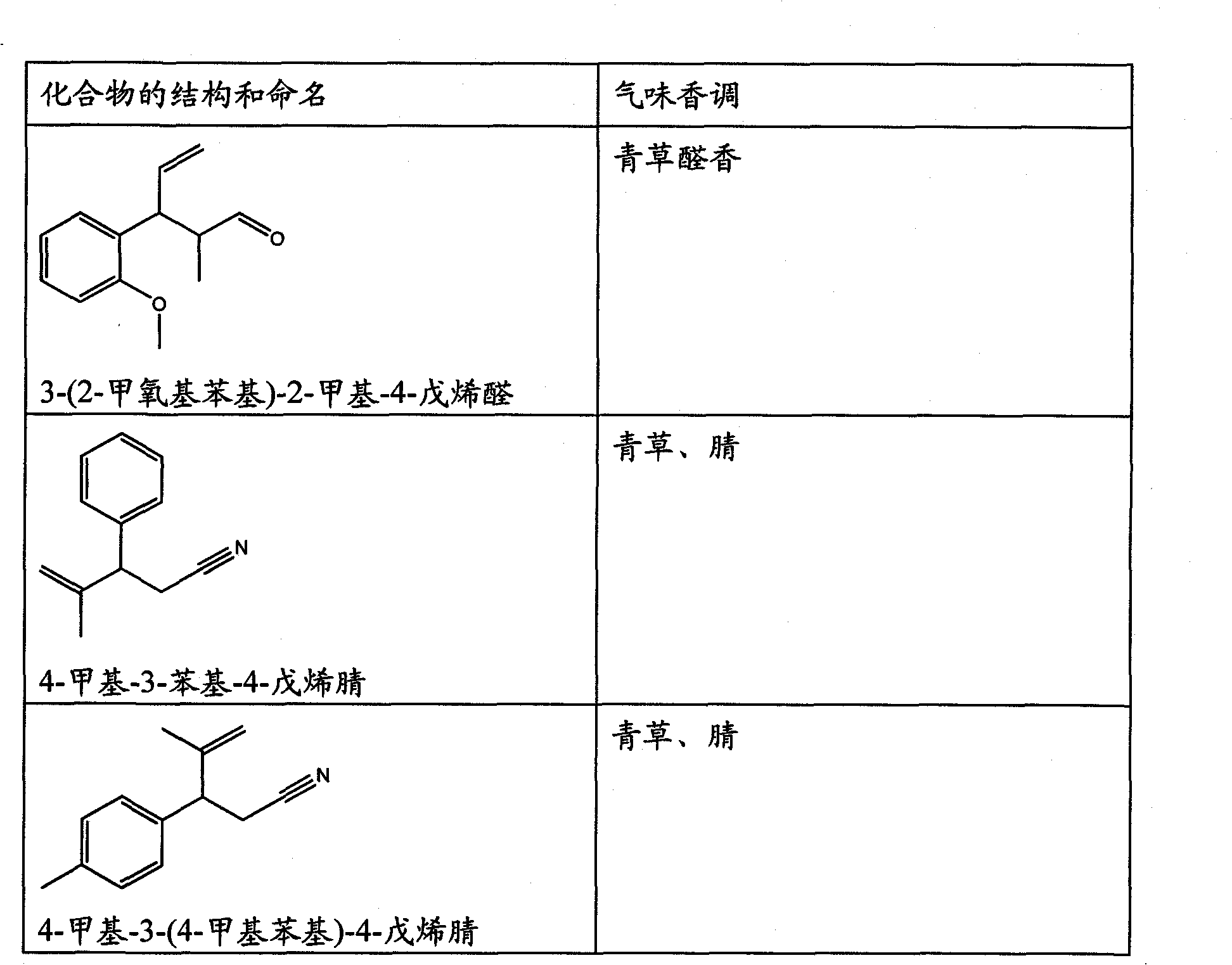
The present invention concerns the use as perfuming ingredient, for instance to impart odor notes of the floral and / or green type, of a compound of formula 5 wherein R is an ortho, meta or para substituent of the phenyl, and represents a hydrogen atom or a C 1-2 alkyl or alkoxyl group; R 1 represents a hydrogen atom or a methyl or ethyl group; 10 R 2 represents a hydrogen atom or a C 1-3 alkyl group; and X represents a CHO, COOR 3, CH(OR 4) 2 or CN group, R 3 being a methyl or ethyl group, and R 4, taken seperately, being a methyl or ethyl group, or said R 4, taken together, a C 2-5 alkanediyl group; and at least one of said R, R 1 or R 2 represents a group containing at least one carbon atom.
Owner:FIRMENICH SA
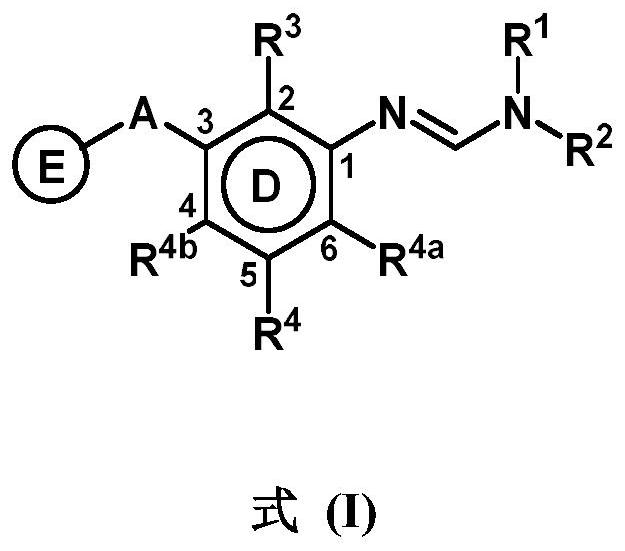
3-substituted phenylamidine compounds, preparation and use thereof
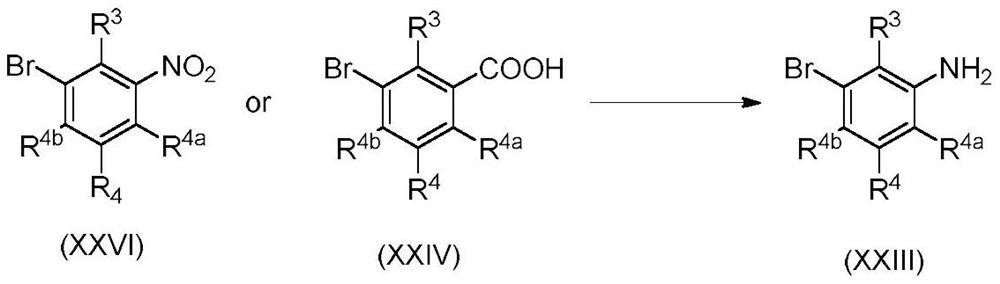
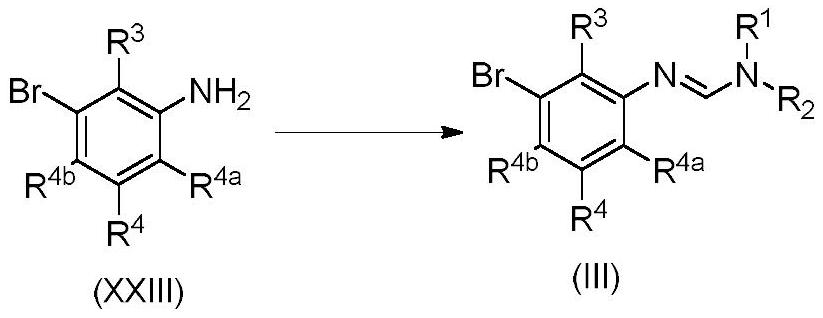
PendingUS20220089523A1Enhanced activity against microbialsWide applicationBiocideAmino preparation from aminesMedicinal chemistryCrop protection
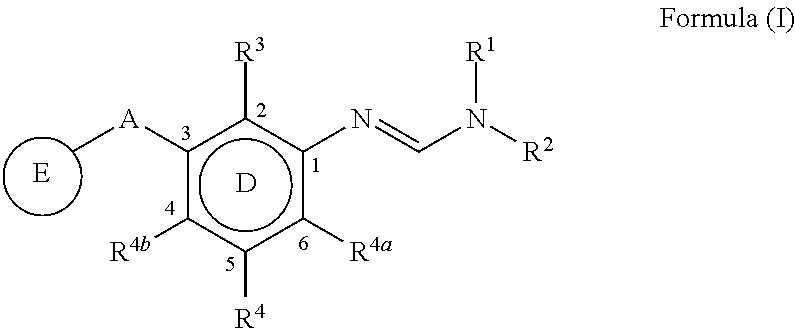
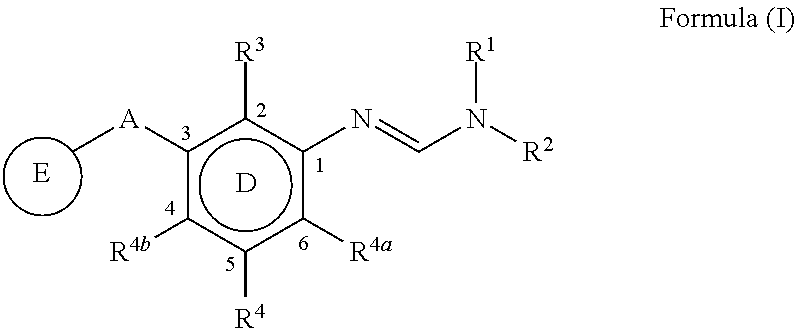
The present invention disclosed 3-substituted phenylamidine compounds of general formula (I), wherein R1, R2, R3, R4, R4a, R4b, A and E have the same meanings as defined in description. The present invention further discloses methods for their preparation and use of the compounds of general formula (I) as a crop protection agent.
Owner:PI IND LTD
Industrialized method for producing 2, 6-dichloro phenyl nitrile
InactiveCN1304367CBiocidePreparation by nitrogen oxide-organic compound reactionMedicinal chemistryHydroxylammonium chloride
The invention relates to a method for industrialized producing 2.6-dichlorobenzonitrile, adopting 6-chloro-o-nitrotoluene chloro-o-nitrotoluene as raw material to make chlorination reaction under the action of catalyst, then making cyanogenation reaction with formic acid and hydroxylammonium chloride, and refining again and again to obtain 2.6-dichlorobenzonitrile whose content is 99.8% above. The produced 2.6- dichlorobenzonitrile is a white powdery crystal, purity 99.8%-9995%, melting point 141.0 deg.C -144.0 deg.C, water ratio <=01%, and product yield up to 64%.
Owner:YANGZHOU TIANCHEN FINE CHEM
Production device for preparing 2, 6-dichlorobenzonitrile
ActiveCN103382165BReduce consumptionReasonable designPreparation by nitrogen oxide-organic compound reactionBuffer tankNitrogen
The invention discloses a production device for preparing 2, 6-dichlorobenzonitrile, and relates to the technical field of production of chemically synthesized 2, 6-dichlorobenzonitrile. The production device comprises an evaporator, a buffer tank, a hydrogen chloride recycling tank, a chlorination kettle, a first rectifying still and a hydrolysis quaternizing kettle. The production device is reasonable in design, convenient to control, less in raw material consumption and low in production cost, solid waste is decreased, emission of nitrogenous nitroso-group waste gas is reduced, and the production device is an energy-saving, emission-reduction and environment-friendly industrial practical cleaning production technique.
Owner:永椿化工新材料有限公司
Process for producing organic compound using nitrite
A process produces an organic compound by allowing (A) a compound capable of generating a free radical to react with (B) at least one of esters and salts of nitrous acid in the presence of a nitrogen-containing cyclic compound constitutively having a skeleton represented by following Formula (i) in its ring: <CHEM> wherein X is an oxygen atom or an -OR group, and wherein R is a hydrogen atom or a hydroxyl-protecting group. Examples of the nitrogen-containing cyclic compound are cyclic imide compounds having a cyclic imide skeleton represented by following Formula (I): <CHEM> wherein n is 0 or 1; X is an oxygen atom or an -OR group, and wherein R is a hydrogen atom or a hydroxyl-protecting group.A process produces an organic compound by allowing (A) a compound capable of generating a free radical to react with (B) at least one of esters and salts of nitrous acid in the presence of a nitrogen-containing cyclic compound constitutively having a skeleton represented by following Formula (i) in its ring: <CHEM> wherein X is an oxygen atom or an -OR group, and wherein R is a hydrogen atom or a hydroxyl-protecting group. Examples of the nitrogen-containing cyclic compound are cyclic imide compounds having a cyclic imide skeleton represented by following Formula (I): <CHEM> wherein n is 0 or 1; X is an oxygen atom or an -OR group, and wherein R is a hydrogen atom or a hydroxyl-protecting group.
Owner:DAICEL CHEM IND LTD
A kind of preparation method of p-aminobenzamidine hydrochloride
InactiveCN105330568BEasy to operateEasy to controlPreparation by nitrogen oxide-organic compound reactionP-aminobenzamidineBenzamidine hydrochloride
The invention discloses a preparation method for p-aminobenzamidine hydrochloride, and belongs to the field of drug synthesis. The preparation method comprises the following steps: taking p-aminobenzamidine as a starting raw material, and enabling the p-aminobenzamidine and hydroxylamine hydrochloride to generate nitrobenzonitrile; then, enabling the nitrobenzonitrile to react with ammonium salt to generate p-aminobenzamidine; carrying out acylation and reduction on the p-aminobenzamidine to obtain p-aminobenzamidine imidogen n-hexyl formate; finally, performing salifying on the p-aminobenzamidine imidogen n-hexyl formate and hydrogen chloride to obtain the p-aminobenzamidine hydrochloride. Compared with the prior art, the preparation method for the p-aminobenzamidine hydrochloride disclosed by the invention has the characteristics of being simple to operate, easy to control in reaction, low in production cost and the like, and has a very good popularization and application value.
Owner:INST OF PHARMACY SHANDONG PROV ACAD OF MEDICAL SCI
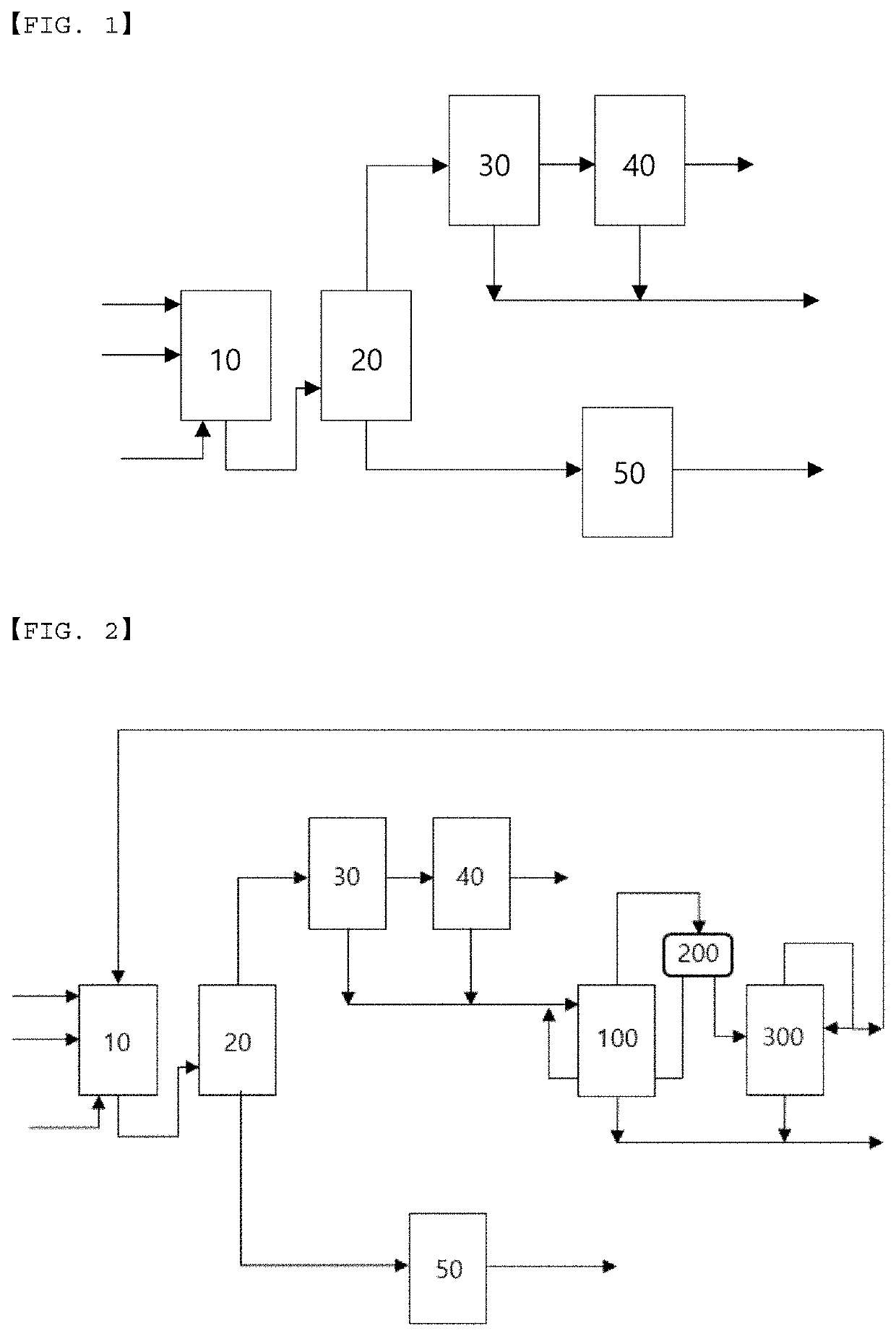
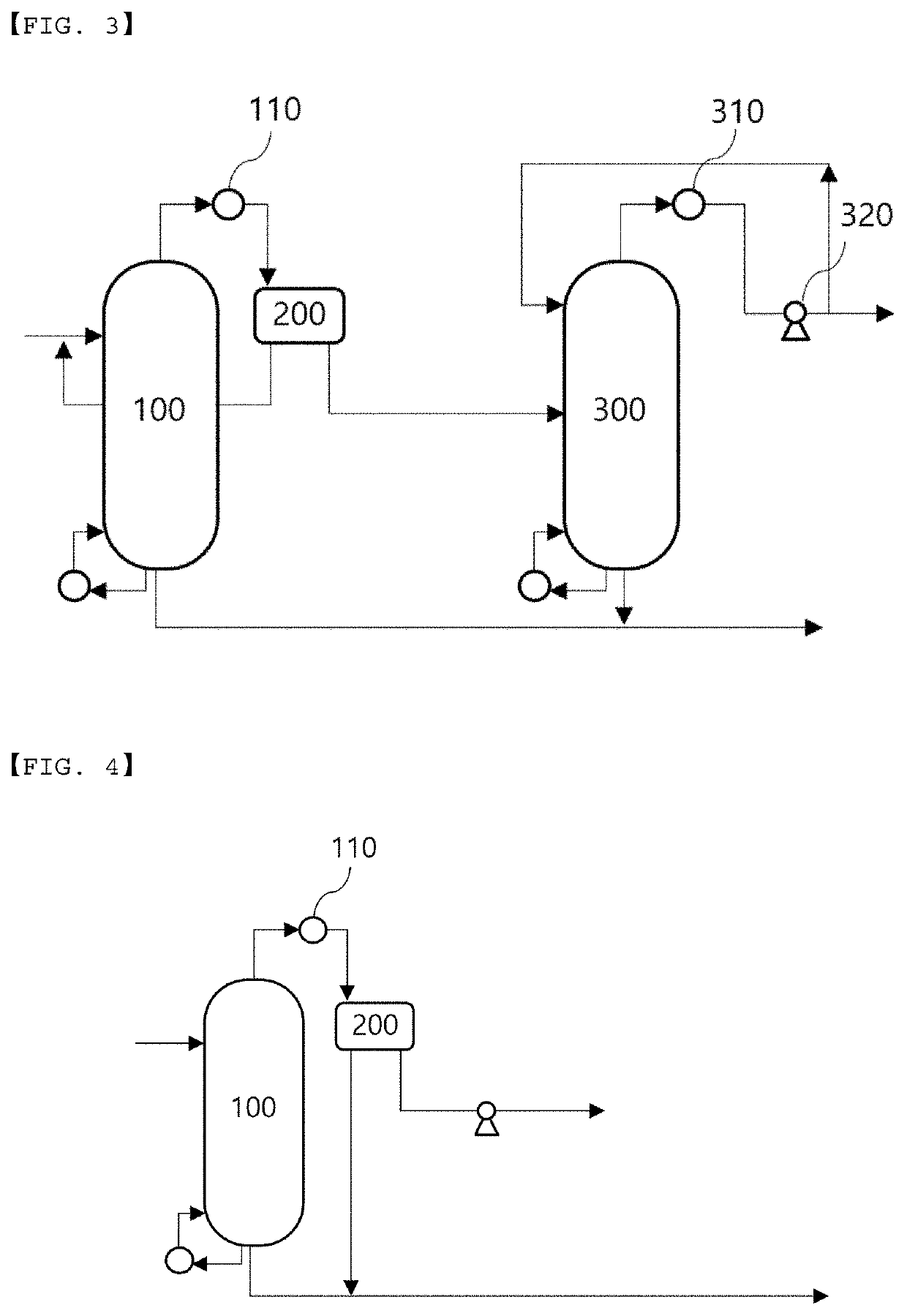
Recovery method and recovery apparatus of nitrile-based monomer
PendingUS20220348536A1High purityReduce loadPreparation by nitrogen oxide-organic compound reactionDistillation separationDistillationProcess engineering
Disclosed is a recovery method of a nitrile-based monomer including: supplying a feed stream including a nitrile-based monomer, a nitrogen compound, and water to a first distillation tower to separate the stream into a lower discharge stream and an upper discharge stream; condensing the upper discharge stream of the first distillation tower and supplying the condensed stream to a decanter to separate the stream into a water layer and an organic layer; supplying an organic layer stream discharged from the decanter to a second distillation tower to separate the stream into a lower discharge stream and an upper discharge stream; and splitting a part of the upper discharge stream from the second distillation tower and refluxing the split stream to the second distillation tower.
Owner:LG CHEM LTD
3-substituted phenylamidine compounds, preparation and use thereof
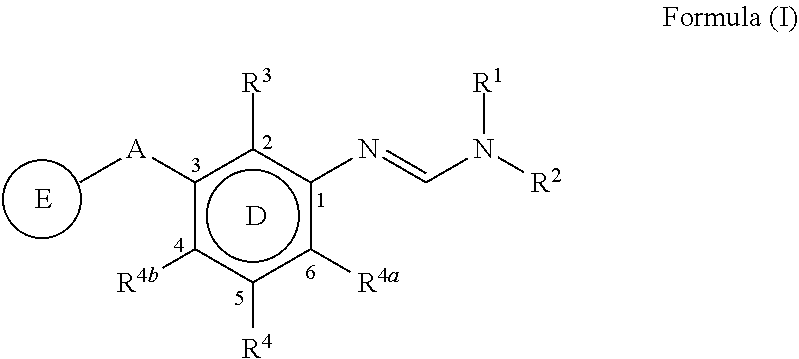
PendingCN113454063AEnhanced antimicrobial activityBiocideAmino preparation from aminesMedicinal chemistryCrop protection
The present invention disclosed 3-substituted phenylamidine compounds of general formula (I), wherein R1, R2, R3, R4, R4a, R4b, A and E have the same meanings as defined in description. The present invention further discloses methods for their preparation and use of the compounds of general formula (I) as a crop protection agent.
Owner:PI IND LTD
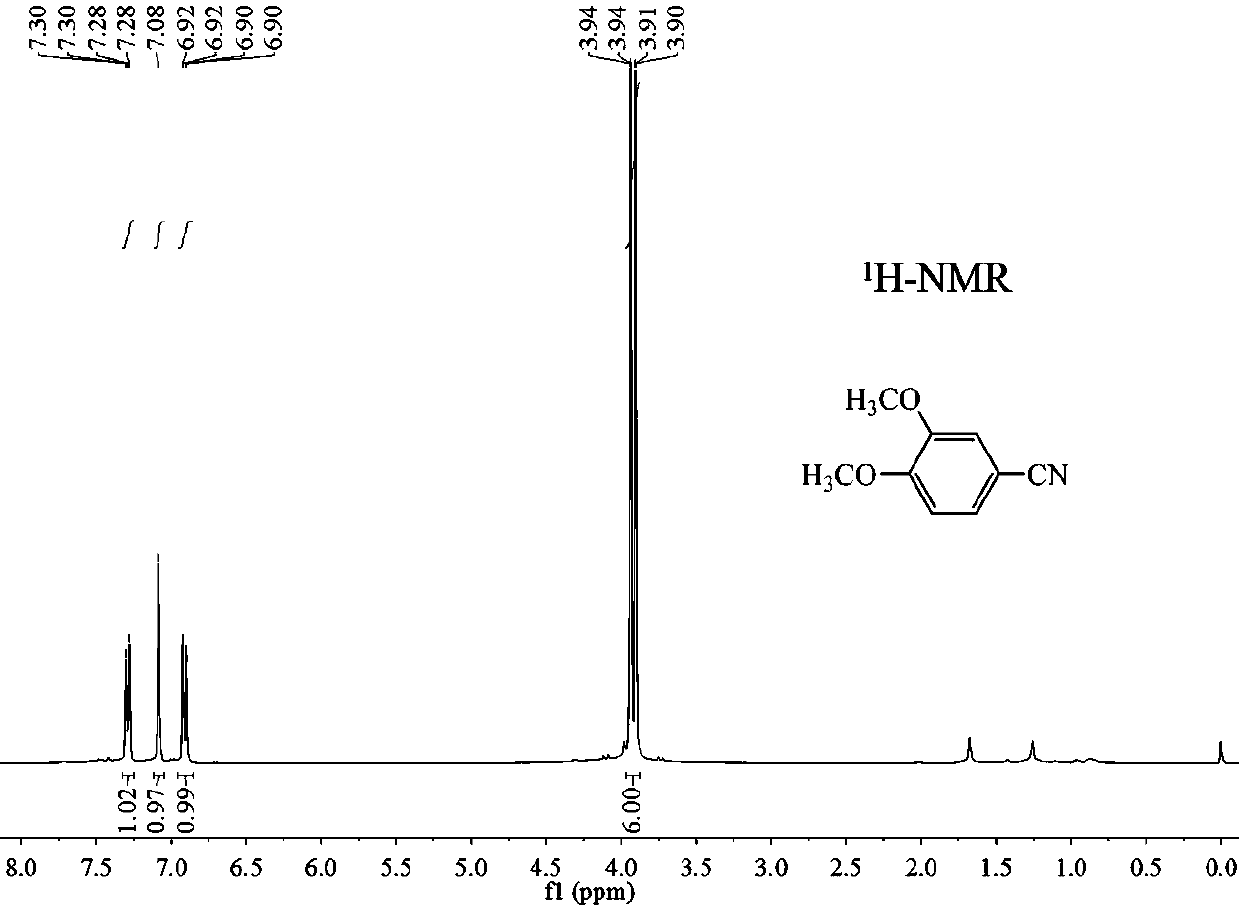
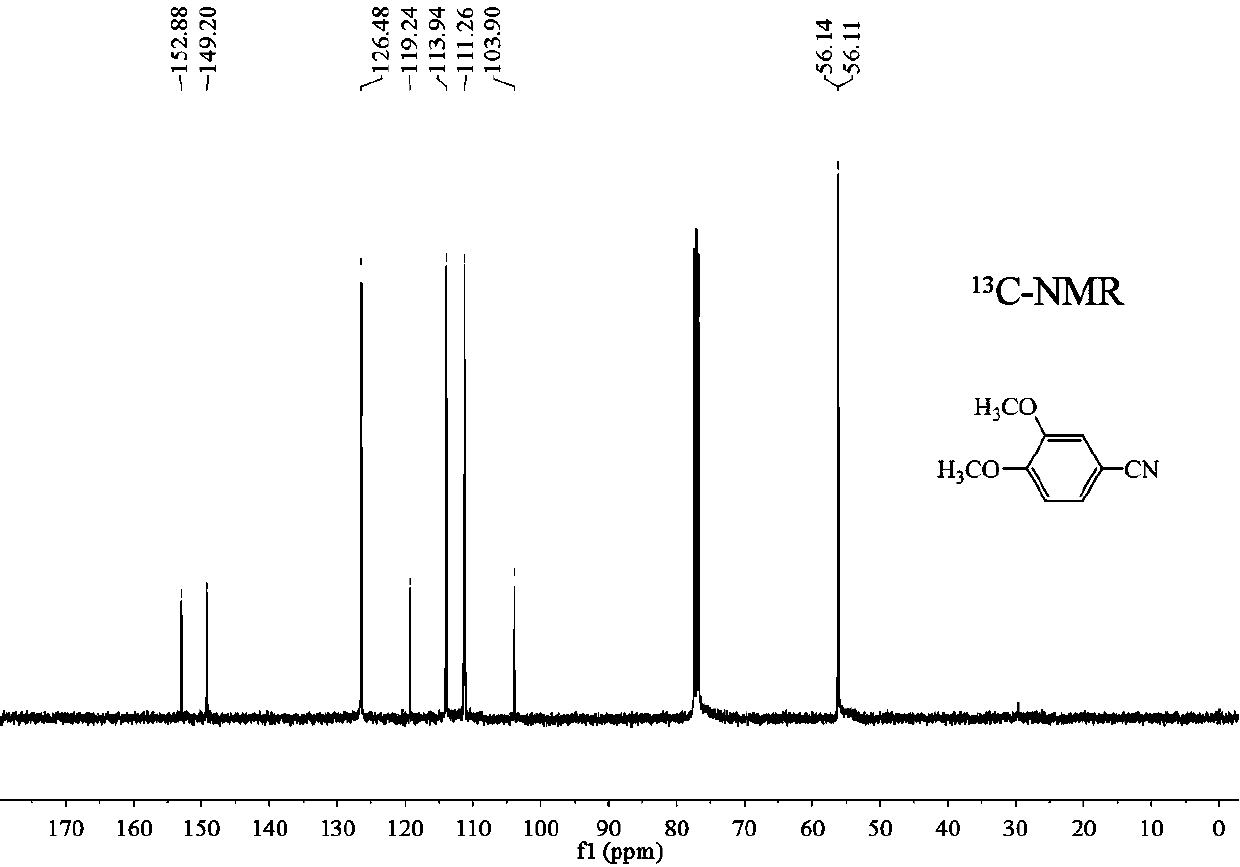
Preparation method of 3, 4-dimethoxybenzonitrile
InactiveCN111302970ABroaden your optionsReduce pollutionPreparation by nitrogen oxide-organic compound reactionOrganic solventNitrogen oxides
The invention provides a preparation method of 3, 4-dimethoxybenzonitrile, which comprises the following steps of adding 3, 4-dimethoxyphenyl acetone and nitrogen oxide into an organic solvent, and carrying out catalytic reaction by an iron catalyst under the protection of inert gas to obtain the 3, 4-dimethoxybenzonitrile. According to the method, 3, 4-dimethoxyphenyl acetone is used as a raw material, so that high yield can be realized at a relatively mild reaction temperature.
Owner:HENAN AGRICULTURAL UNIVERSITY
Method and mixture to form functionalized cyclic compounds
ActiveUS11053189B2Effective and low-cost and environmentally friendlyImprove responseOrganic compound preparationCarboxylic acid amides preparationMoietyCyclic compound
Owner:NATIONAL TSING HUA UNIVERSITY
Popular searches
Catalytic reactions Preparation by hydrogen cyanide addition Controllers with particular characteristics Animal repellants Herbicides and algicides Plant growth regulators Amino compound preparation Carboxylic acid nitrile purification/separation Carboxylic acid esters preparation Preparation by cyanide reaction
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com