Process for producing organic compound using nitrite
A technology for organic compounds and nitroso compounds, which is applied in the preparation of organic compounds, organic chemical methods, and preparation of nitro compounds, and can solve problems such as low space-time yields and harsh reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
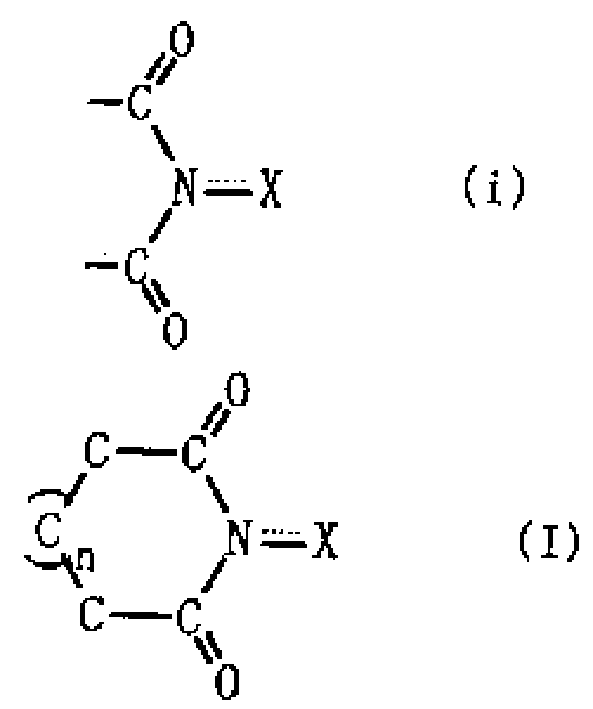
Image
Examples
Embodiment 1
[0118] Cyclopentane (1ml), tert-butyronitrile (1mmol), N-hydroxyphthalimide (0.2mmol) and acetic acid (1ml) were put into a flask and heated under an argon atmosphere (1atm=0.101 MPa) for 20 hours. Analysis of the resulting reaction mixture revealed formation of cyclopentanone oxime, nitrocyclopentane, cyclopentanone and cyclopentyl acetate in yields of 4%, 2%, 3% and less than 1%, respectively.
Embodiment 2
[0120] Cyclohexane (1ml), tert-butyronitrile (1mmol), N-hydroxyphthalimide (0.2mmol) and acetic acid (1ml) were put into the flask and heated under an argon atmosphere (1atm=0.101 MPa) for 20 hours. Analysis of the resulting reaction mixture revealed the formation of cyclohexanone oxime, nitrocyclohexane, cyclohexanone and cyclohexyl acetate in yields of 16%, 10%, 3% and 2%, respectively.
Embodiment 3
[0122] Cyclohexane (1ml), tert-butyronitrile (1mmol), 4-chloro-N-hydroxyphthalimide (0.2mmol) and acetic acid (1ml) were put into a flask and heated at 80°C under an argon atmosphere (1 atm = 0.101 MPa) and stirred for 20 hours. Analysis of the resulting reaction mixture revealed the formation of cyclohexanone oxime, nitrocyclohexane, cyclohexanone and cyclohexyl acetate in yields of 13%, 9%, 2% and 2%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com