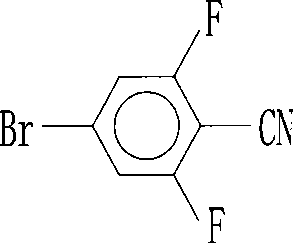
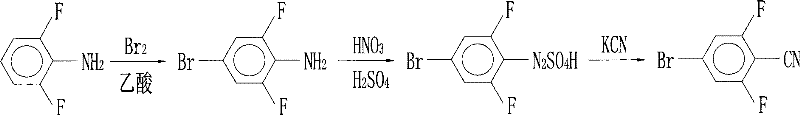
Preparation of 4-bromo-2,6-difluorobenzonitrile
A technology of difluorobenzonitrile and difluorobromobenzene, applied in the direction of nitrogen oxide-organic compound reaction preparation, organic chemistry, etc., can solve the problems of high cost, high toxicity, serious environmental pollution, etc., and achieve the convenience of mass production and low price Inexpensive, less pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0041] The present invention will be further described below.
[0042] 1. Preparation of 4-bromo-2,6-difluorobenzaldehyde
[0043] The raw materials used for preparing 4-bromo-2,6-difluorobenzaldehyde are: tetrahydrofuran (technical grade), potassium tert-butoxide, petroleum ether (technical grade), 3,5-difluorobromobenzene (gas chromatography content GC≥ 99%), DMF (i.e. N, N-dimethylformamide), butyllithium (density of 2.4mol / L); the material ratio of the main raw materials should follow: butyllithium / 3,5-difluorobromobenzene =1.2mol / 1mol, DMF / 3,5-difluorobromobenzene=1.6mol / 1mol; the instruments used in the preparation process mainly include: 5-liter low-temperature kettle, -150~100℃ low-temperature thermometer, constant pressure dropping funnel , nitrogen cylinders, water circulation pumps, liquid nitrogen tanks, butyllithium storage tanks, etc.
[0044] The process of preparing 4-bromo-2,6-difluorobenzaldehyde comprises the following steps:
[0045] Step 1 Preparation: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com