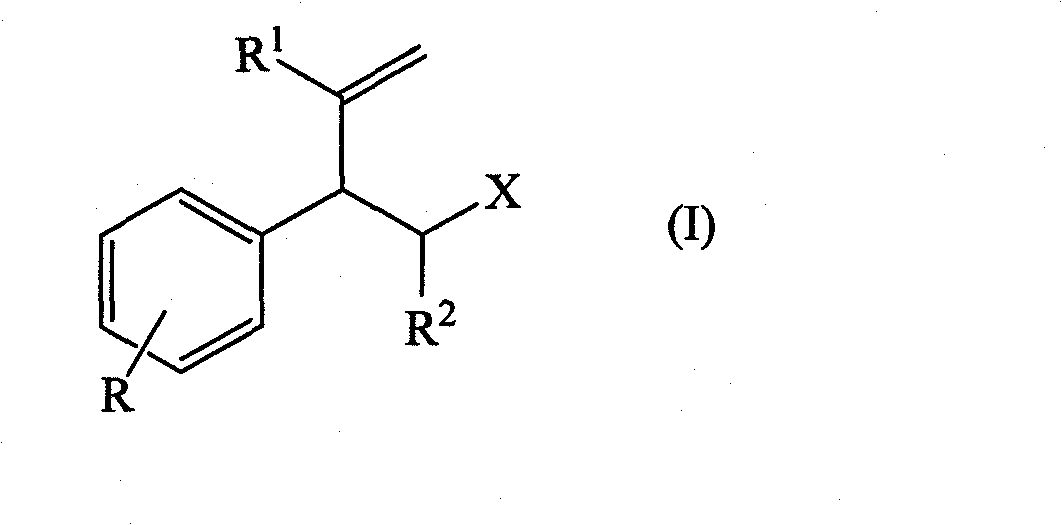
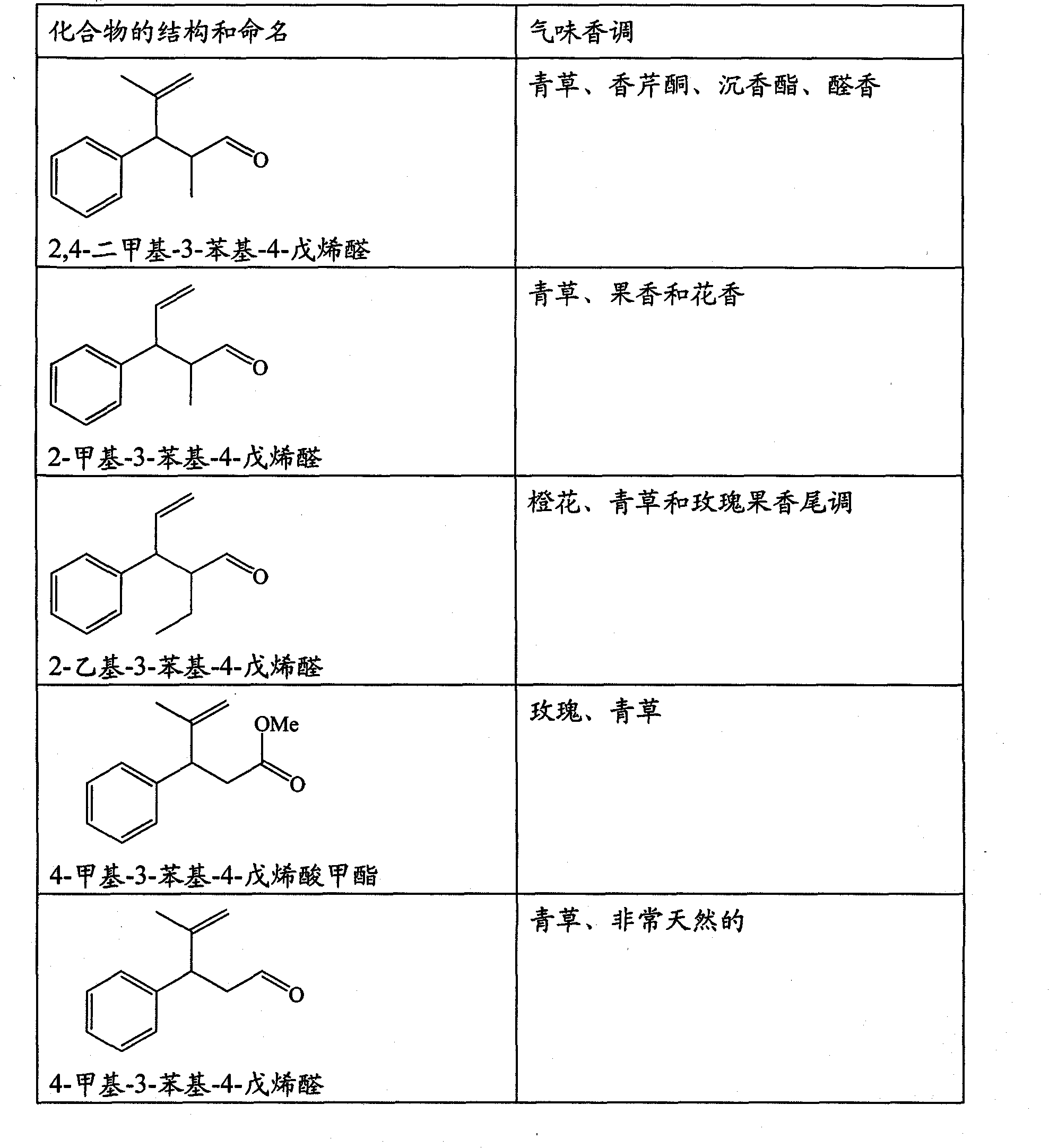
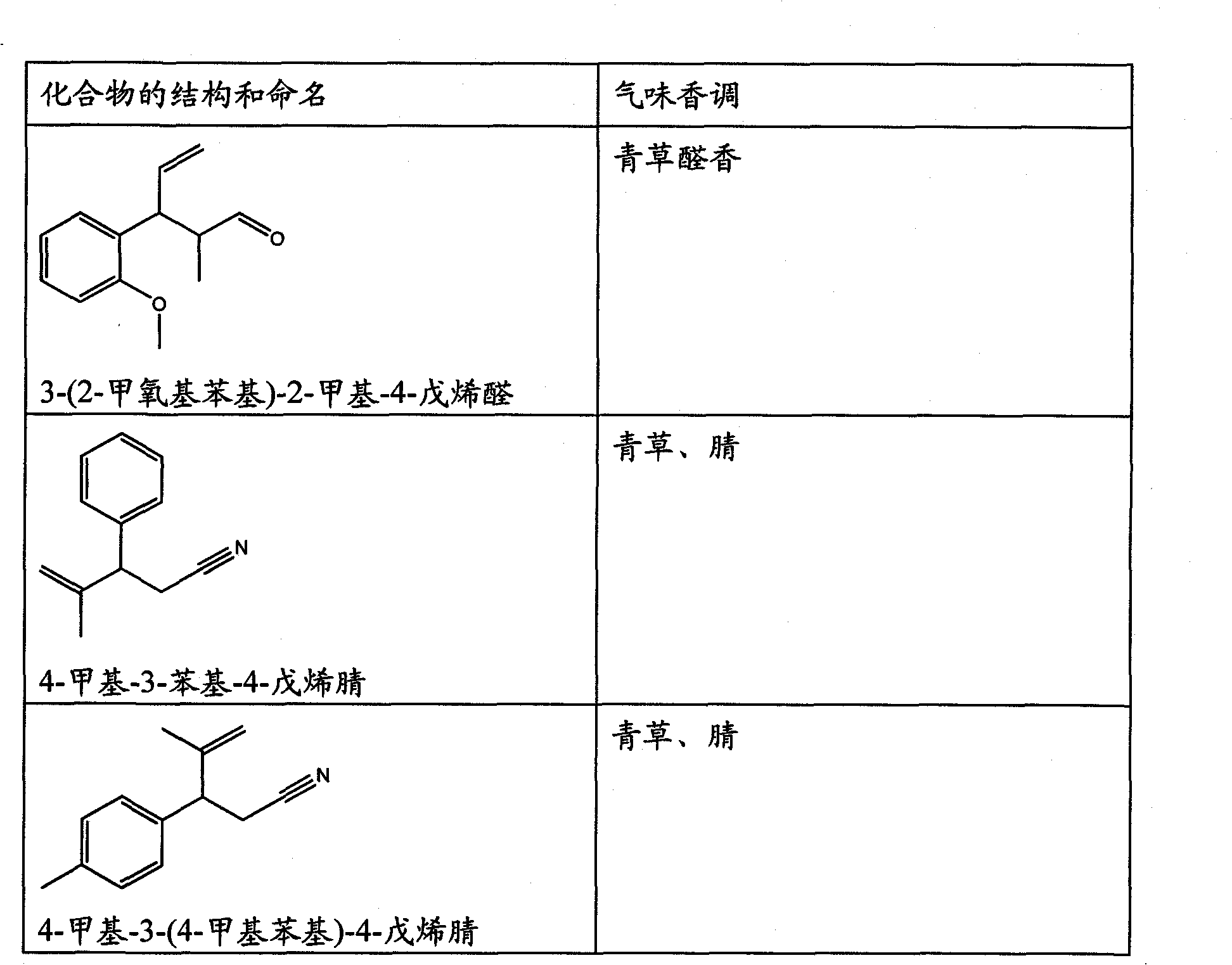
Perfuming ingredients of the floral and/or anis type
A compound, methyl technology, applied in the field of C12-C17 substituted derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Synthesis of compounds of general formula (I)
[0074] 1-[(1E)-3-(allyloxy)-2-methyl-1-propenyl]-4-methylbenzene
[0075] Solid potassium tert-butyrate (47 g, 0.411 mol) was added portionwise to E-3-(4-methylphenyl)-2-methyl-2-propen-1-ol (68.05 g, 0.420mol) in dry THF (800ml) solution (exotherm to 30°C). After 1 hour at room temperature, the reaction was cooled to 5°C and tetrabutylammonium iodide (7.9 g, 0.021 mol) was added dropwise followed by allyl bromide (102.65 g, 0.840 mol). The reaction was warmed to room temperature overnight and poured into water (800ml). The reaction was extracted twice with ethyl acetate. Each organic phase was washed with water and brine. The combined extracts were dried over solid anhydrous sodium sulfate. The solid was filtered off, washed with diethyl ether and the solvent was removed under vacuum. The product was purified by distillation under vacuum through a 20 cm Widmer column. 79 g of the desired product were obtained (yi...
Embodiment 2
[0153] Preparation of Perfuming Compositions
[0154] A musk-herbal type cologne for men is prepared by mixing the following ingredients:
[0155]
[0156]
[0157] * in dipropylene glycol
[0158] ** in isopropyl myristate
[0159] 1) 8,12-Epoxy-13,14,15,16-destetramethylrebadan; source: Firmenich SA, Geneva, Switzerland
[0160] 2) 3-(4 / 2-ethylphenyl)-2,2-dimethylpropanal; source: International Flavors & Fragrances, USA
[0161] 3) 1,3,4,6,7,8-Hexahydro-4,6,6,7,8,8-hexamethyl-cyclopenta-g-2-benzopyran; source: International Flavors & Fragrances, USA
[0162] 4) cis-methyl dihydrojasmonate; source: Firmenich SA, Geneva, Switzerland
[0163] 5) (1S,1'R)-2-[1-(3',3'-dimethyl-1'-cyclohexyl)ethoxy]-2-methylpropyl propionate; source: Firmenich SA , Switzerland, Geneva
[0164] 6) 1-(Octahydro-2,3,8,8-tetramethyl-2-naphthyl)-1-ethanone; source: International Flavors & Fragrances, USA
[0165] 7) 3-(4-tert-Butylphenyl)-2-methylpropanal; Source: Givaudan SA, Geneva,...
Embodiment 3
[0170] Preparation of Perfuming Compositions
[0171] Prepare a floral-water cologne by mixing the following ingredients:
[0172]
[0173]
[0174] * in dipropylene glycol
[0175] ** in isopropyl myristate
[0176] 1) 2,6-Dimethyl-2-heptanol; source: Givaudan SA, Geneva, Switzerland
[0177] 2) Cyclopentadecanolide; Source: Firmenich SA, Geneva, Switzerland
[0178] 3) 1,3,4,6,7,8-Hexahydro-4,6,6,7,8,8-hexamethyl-cyclopenta-g-2-benzopyran; source: International Flavors & Fragrances, USA
[0179] 4) Methyl dihydrojasmonate; source: Firmenich SA, Geneva, Switzerland
[0180] 5) 1-(Octahydro-2,3,8,8-tetramethyl-2-naphthyl)-1-ethanone; source: International Flavors & Fragrances, USA
[0181] 6) 3-(4-tert-Butylphenyl)-2-methylpropanal; Source: Givaudan SA, Geneva, Switzerland
[0182] 7) 4 / 3-(4-Hydroxy-4-methylphenyl)-3-cyclohexene-1-carbaldehyde; source: International Flavors & Fragrances, USA
[0183] 8) (1R)-cis-3-oxo-2-pentyl-1-cyclopentaneacetic acid (+)-me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com