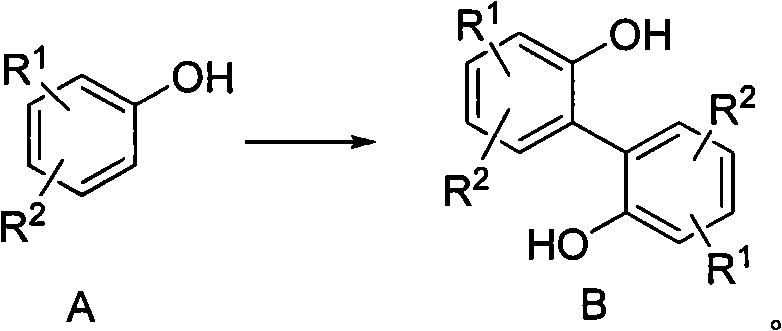
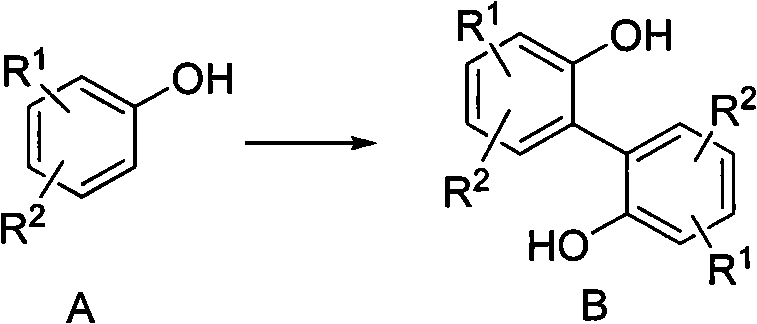
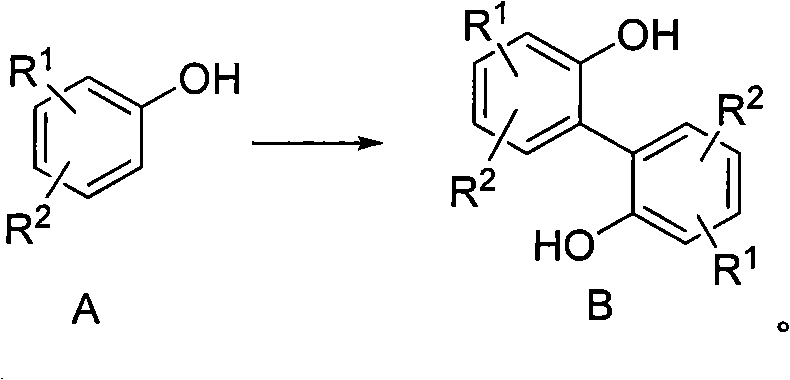
Preparation method of 2, 2'-biphenyl diphenol and derivatives thereof
A technology for biphenol and derivatives, which is applied in the field of preparation 2, can solve the problems of reactor and agitator pollution, complicated post-processing, noise pollution, etc., and achieves the effect of a simple and economical synthesis method and simple and easy post-processing.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 50g (300mmol, 1eq) of 4-methyl-2-tert-butylphenol and 8.11g (150mmol, 0.5eq) of ferric trichloride were added to 250mL of dioxane, and then 31mL (27.4g , 300mmol, 1eq) tert-butyl hydroperoxide, dripped in 40 minutes. After the dropwise addition, the system was heated to 80° C. for 4 hours to react. After the reaction, the dioxane was distilled off under reduced pressure, and the residue was recrystallized from petroleum ether to obtain 44.0 g of 2,2'-di-tert-butyl-4,4'-dimethylbiquinone, with a yield of 90%. . H NMR spectrum ( 1 H NMR, 300MHz, CDCl 3 )δ7.14-7.12 (d, J m =1.25Hz, 2H); 7.00-6.88(d, J m = 1.25 Hz, 2H); 5.18 (s, 2H); 2.30 (s, 6H); 1.242 (s, 18H). MS: m / z 326 (M+); 311; 255.
Embodiment 2
[0040] 77g (500mmol, 1eq) of 3,4-dimethoxyphenol and 17.4g (62.5mmol, 0.125eq) of ferrous sulfate heptahydrate were added to 400mL of toluene, and then 26mL (22.9g, 250mmol, 0.5eq) tert-butyl hydroperoxide, dripped in 30 minutes. After the dropwise addition, the system was heated to 85° C. for 5 hours to react. After the reaction, the dioxane was distilled off under reduced pressure, and the residue was subjected to petroleum ether column chromatography to obtain 62.0 g of 3,3',4,4'-tetramethoxybiquinone with a yield of 81%. H NMR spectrum ( 1 H NMR, 300MHz, CDCl 3 ) δ 7.08 (s, 2H); 6.49 (s, 2H); 5.35 (s, 2H); 3.88 (s, 6H); 3.75 (s, 6H). MS: m / z 306 (M+).
Embodiment 3
[0042] Add 170g (1mol, 1eq) 4-phenylphenol and 40.5g (0.25mol, 0.25eq) ferric trichloride to 850mL ethyl acetate respectively, then add 220mL (175.2g, 1.2mol, 1.2eq) dropwise at room temperature ) di-tert-butyl peroxide, dripped in 150 minutes. After the dropwise addition, the system was heated to 75° C. for 6 hours. After the reaction, ethyl acetate was distilled off under reduced pressure, and the residue was subjected to petroleum ether column chromatography to obtain 118.3 g of 4,4'-diphenylbiphenyldiphenol, with a yield of 70%. H NMR spectrum ( 1 H NMR, 300MHz, CDCl 3 )δ7.58-7.41 (m, 14H); 7.07 (d, J o = 6.2 Hz, 2H); 5.12 (s, 2H). MS: m / z 338 (M+).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com