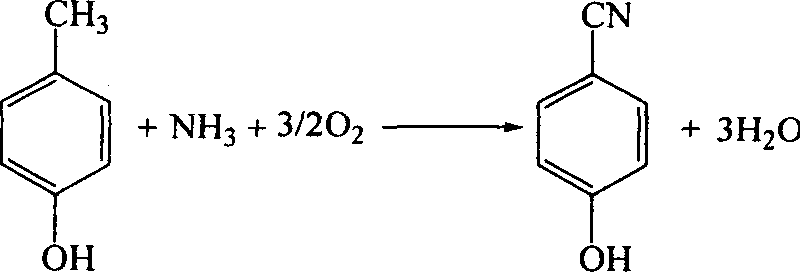Catalyst for synthesizing para-hydroxy-benzonitrile and its preparing method and use
A technology for p-hydroxybenzonitrile and catalyst is applied to the catalyst for synthesizing p-hydroxybenzonitrile and the fields of preparation and use thereof, which can solve problems such as lack of catalyst stability, and achieve small molar ratio, selectivity and yield The effect of high and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Catalyst preparation
[0026] 3.6gCrO 3 , 1.2g (NH 4 ) 6 Mo 7 o 24 4H 2 O and 33.8g H 3 PO 4 Add deionized water to prepare a solution, and then add 3.1g FeCl to the solution 3 ·6H 2 O, 1.5 g Na 2 O, 0.8gK 2 O was carefully heated to make it a transparent solution, and then 40 g of calcined fine-grained SiO 2 (produced by Qingdao Ocean Chemical Co., Ltd.) was added to the above transparent solution, stirred evenly, dried at 373K, and then activated at 823K for 6 hours to obtain the required catalyst.
[0027] P-Hydroxytoluene Ammoxidation
[0028] In a quartz tube reactor with an internal diameter of 40mm, add 80ml of catalyst, after the temperature is raised to 633K, the reaction mixture is continuously sent into the reaction mixture at a molar ratio of p-cresol: ammonia: air: steam = 1: 1.2: 20: 10. and collect the reaction mixture from the outlet. After the catalyst was used continuously for 50...
Embodiment 2
[0030] Catalyst preparation
[0031] 6.5gCrO 3 , 1.5g (NH 4 ) 6 Mo 7 o 24 4H 2 O and 50.1 g H 3 PO 4Add deionized water to prepare a solution, and then add 4.1g K to the solution 2 O, 6.1 g FeCl 3 ·6H 2 O was carefully heated to make it a transparent solution, and then 40 g of calcined fine-grained SiO 2 (produced by Qingdao Ocean Chemical Co., Ltd.) was added to the above transparent solution, stirred evenly, dried at 373K, and then activated at 733K for 8 hours to obtain the required catalyst.
[0032] P-Hydroxytoluene Ammoxidation
[0033] In a quartz tube reactor with an internal diameter of 40mm, add 80ml of catalyst, after the temperature is raised to 633K, the reaction mixture is continuously sent into the reaction mixture at a molar ratio of p-cresol: ammonia: air: steam = 1: 1.2: 20: 10. and collect the reaction mixture from the outlet. After the catalyst was used continuously for 50 hours, ...
Embodiment 3
[0035] Catalyst preparation
[0036] 1.8gCrO 3 , 1.2g (NH 4 ) 6 Mo 7 o 24 4H 2 O and 16.1 g H 3 PO 4 Add deionized water to prepare a solution, and then add 0.3g Na to the solution 2 O, 0.75gK 2 O, carefully heated to make it a transparent solution, and then calcined 40g of fine particle SiO 2 (produced by Qingdao Ocean Chemical Co., Ltd.) was added to the above transparent solution, stirred evenly, dried at 433K, and then activated at 753K for 3 hours to obtain the required catalyst.
[0037] P-Hydroxytoluene Ammoxidation
[0038] In a quartz tubular reactor with an internal diameter of 40 mm, add 80 ml of catalyst, and after the temperature is raised to 633 K, the reaction mixture is continuously sent into the reaction mixture at a molar ratio of p-cresol: ammonia: air: water vapor=1: 1.3: 10: 5. and collect the reaction mixture from the outlet. After the catalyst was used continuously for 50 hours,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com