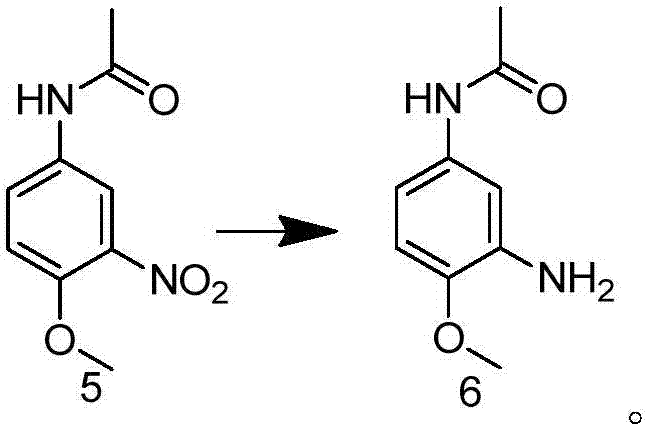
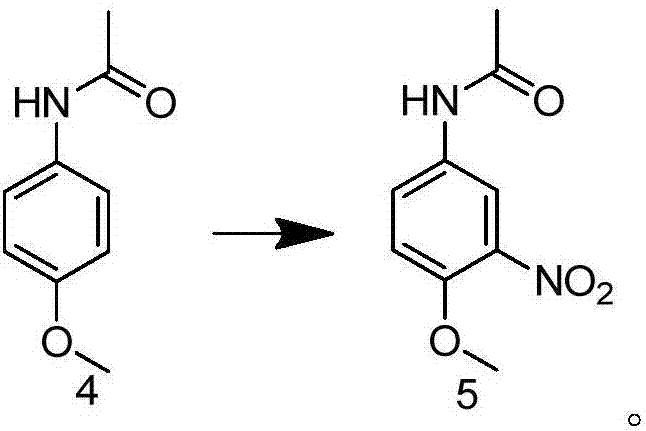
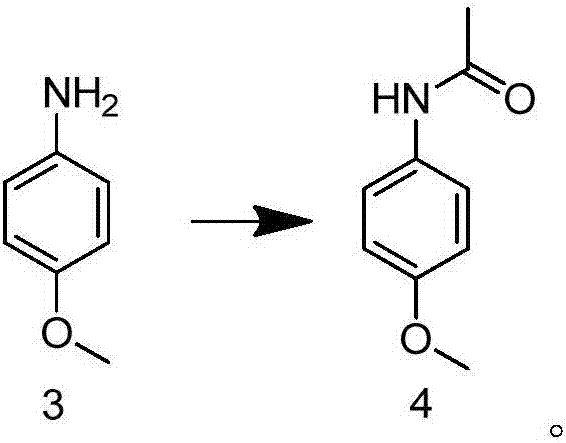
Preparation method of 2-methoxy-5-acetamidoaniline
A technology of acetamidoaniline and methoxy group, applied in the field of preparation of 2-methoxy-5-acetamidoaniline, can solve the problems of poor environmental protection, difficult catalyst or solvent recovery, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] The preparation of embodiment 1 p-nitroanisole
[0079] Add 50 grams of sodium hydroxide and 60 grams of methanol to a 1000ml three-necked bottle, stir and heat up to 55°C, keep warm for 30 minutes to dissolve all the sodium hydroxide, then add 2 grams of tetrabutylammonium bromide, and control the temperature not to exceed 60°C Next, add 50 grams of p-chloronitrobenzene in batches, after the addition is complete, keep warm at 55-60°C for 1 hour, then raise the temperature to 60-70°C and keep warm for 4-5 hours, and control in HPLC until the raw material is less than 0.3%, which is the end of the reaction. Recover part of methanol (30 g under normal pressure), add 200 g of ice water under stirring, a large amount of yellow solid precipitates, filter, wash with water until neutral, and obtain 45 g of p-nitroanisole, yield 92.6%, GC purity 99.0%. The melting point is 53-54°C.
Embodiment 2
[0080] The preparation of embodiment 2 p-nitroanisole
[0081]In a 1000ml three-necked flask, add 40 grams of sodium hydroxide and 60 grams of methanol, stir and heat up to 55°C, keep warm for 30 minutes to dissolve all the sodium hydroxide, then add 2 grams of tetrabutylammonium bromide, and control the temperature not to exceed 60°C Next, add 50 grams of p-chloronitrobenzene in batches, after the addition is complete, keep warm at 55-60°C for 1 hour, then raise the temperature to 60-70°C and keep warm for 4-5 hours, and control in HPLC until the raw material is less than 0.3%, which is the end of the reaction. Recover part of methanol (30 g under normal pressure), add 200 g of ice water under stirring, a large amount of yellow solid precipitates, filter, wash with water until neutral, and obtain 45 g of p-nitroanisole, yield 91%, GC purity 98.9.0%. The melting point is 53-54°C.
[0082] Other experimental conditions and results are shown in the following table: Among them,...
Embodiment 3
[0084] The preparation of embodiment 3 p-nitroanisole
[0085] In a 1000mL three-necked flask, add 50g of sodium hydroxide and 30g of methanol recovered from Example 1 and 30g of new methanol and stir to raise the temperature to 55°C and keep it warm for 30 minutes to completely dissolve the sodium hydroxide, then add 2g of tetrabutylammonium bromide , under the condition that the temperature does not exceed 60°C, add 50 grams of p-chloronitrobenzene in batches, after the addition, keep it at 55-60°C for 1 hour, then raise the temperature to 60-70°C and keep it for 4-5 hours, and control the raw material in HPLC Less than 0.3% is the end point of the reaction. Recover 30 grams under normal pressure, add 200 grams of ice water under stirring, a large amount of yellow solid precipitates, filter, wash with water until neutral, and obtain 44.8 grams of p-nitroanisole, with a yield of 92.4% . Melting point 53-54°C, GC purity 99.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com