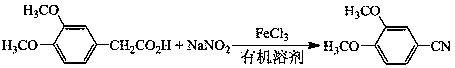Preparation method of 3, 4-dimethoxybenzonitrile
A technology of dimethoxybenzonitrile and dimethoxybenzene, which is applied in the field of preparation of chemical raw materials, can solve the problems of high-energy chemical bond carbon-carbon bond breakage, difficult reaction, etc., achieves low toxicity, simple steps, and reduced process cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Take magneton, 2 mL dimethyl sulfoxide, 0.5 mmol 3,4-dimethoxyphenylacetic acid, 3 mmol sodium nitrite and 0.5 mmol ferric chloride and add them to the glass pressure-resistant reaction tube in turn, after that, the reaction The tube was sealed and placed in a heating tank at a temperature of 50° C., and heated for 10 h under magnetic stirring. After the reaction, the reaction system was cooled to room temperature.
[0027] Afterwards, the internal standard quantitative analysis of the above reaction product was carried out by gas chromatography, and the yield of 3,4-dimethoxybenzonitrile product was measured to be 82%.
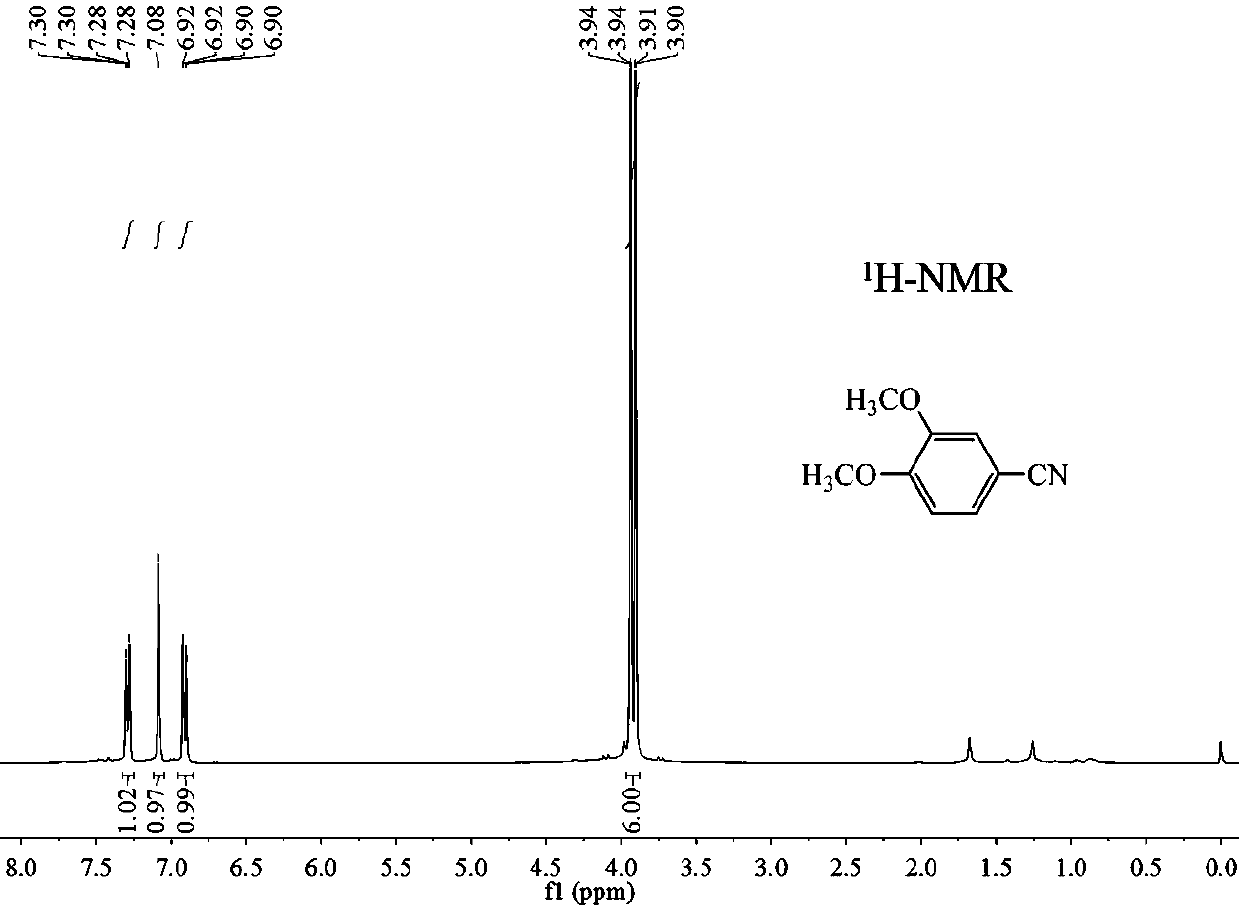
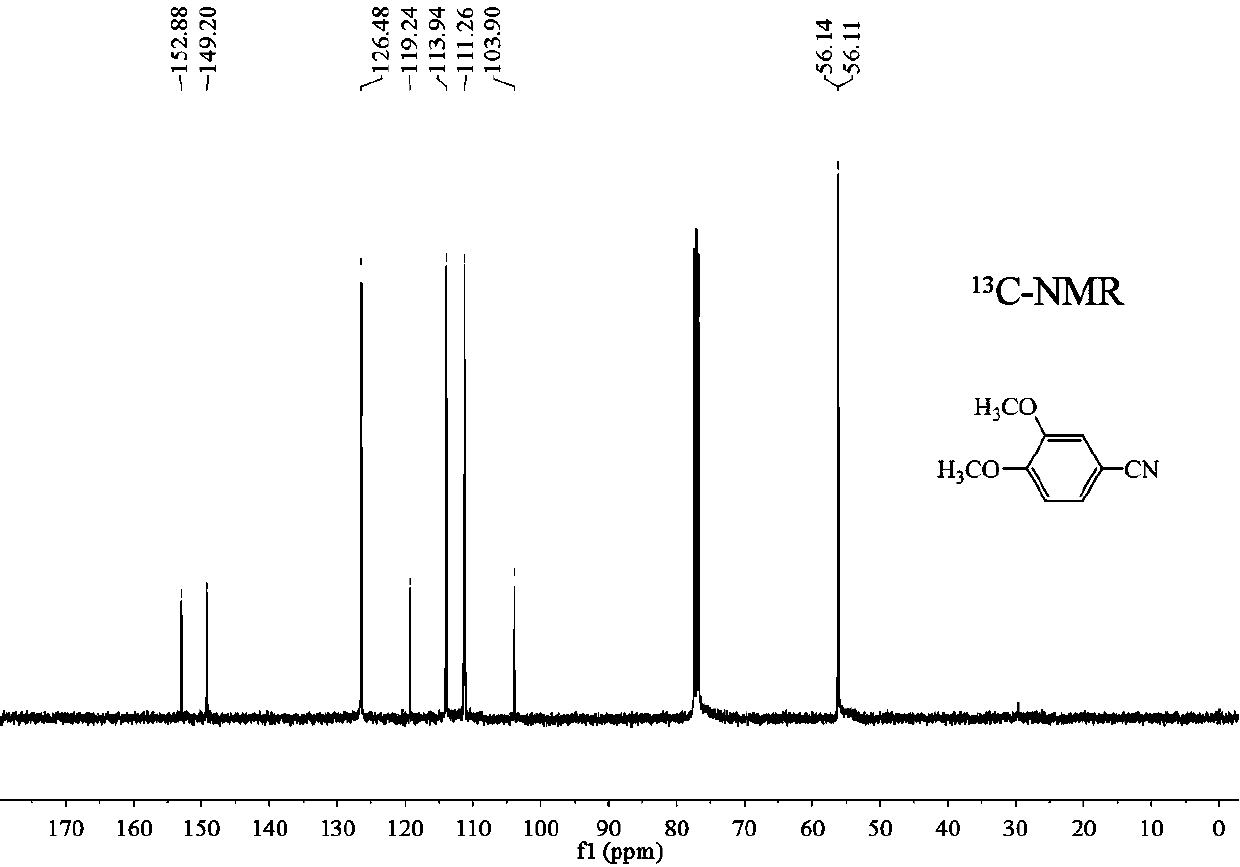
[0028] Then the above experimental steps were repeated, and after the reaction system was cooled to room temperature, the product was separated and purified by column chromatography to obtain the purified 3,4-dimethoxybenzonitrile finished product. use 1 H-NMR, 13 C-NMR confirms the structure of the product in the appendix figure 1 And attached f...
Embodiment 2-7
[0030] Change the consumption of sodium nitrite into 1mmol, 1.5mmol, 2mmol, 2.5mmol, 3.5mmol, 4mmol respectively in the embodiment 1, other conditions are constant, draw the productive rate of product (gas phase internal standard) to be respectively 20%, 51%. %, 70%, 75%, 90%, 89%.
Embodiment 8-11
[0032] Change the consumption of ferric chloride in embodiment 1 into 0.25mmol, 1mmol, 1.5mmol, 2mmol respectively, other conditions are constant, draw the productive rate of product (gas phase internal standard) to be 43%, 82%, 75% respectively , 63%, 47%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com