One-pot aromatic nitrile synthesis method adopting Fe (III) porphyrin for catalyzing nitrite to oxidize aromatic olefin
A technology of porphyrin catalyzing nitrite and nitrite, which is applied in the fields of organic chemical synthesis and pharmaceutical intermediates, can solve the problems of harsh reaction conditions, low yield and the like, and achieves easy controllable operation, reduced production cost, and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
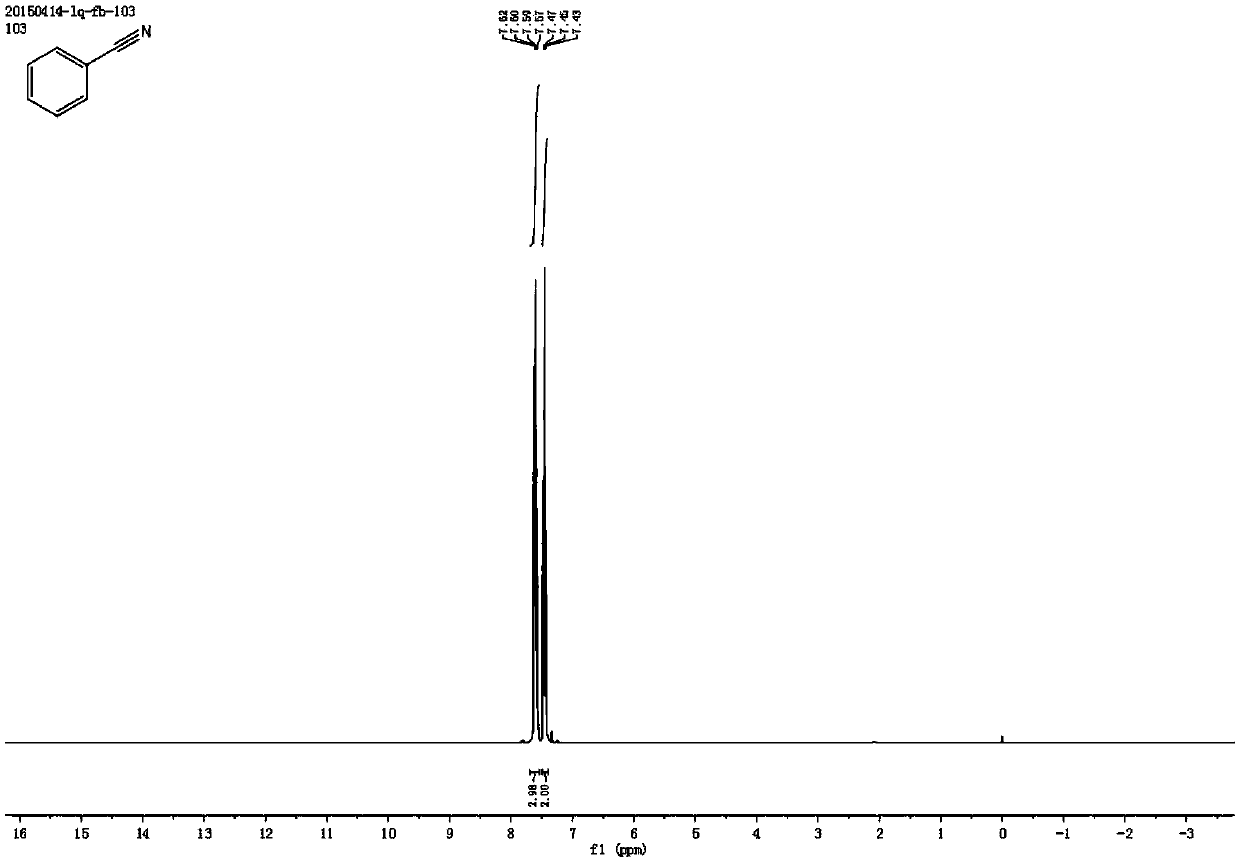
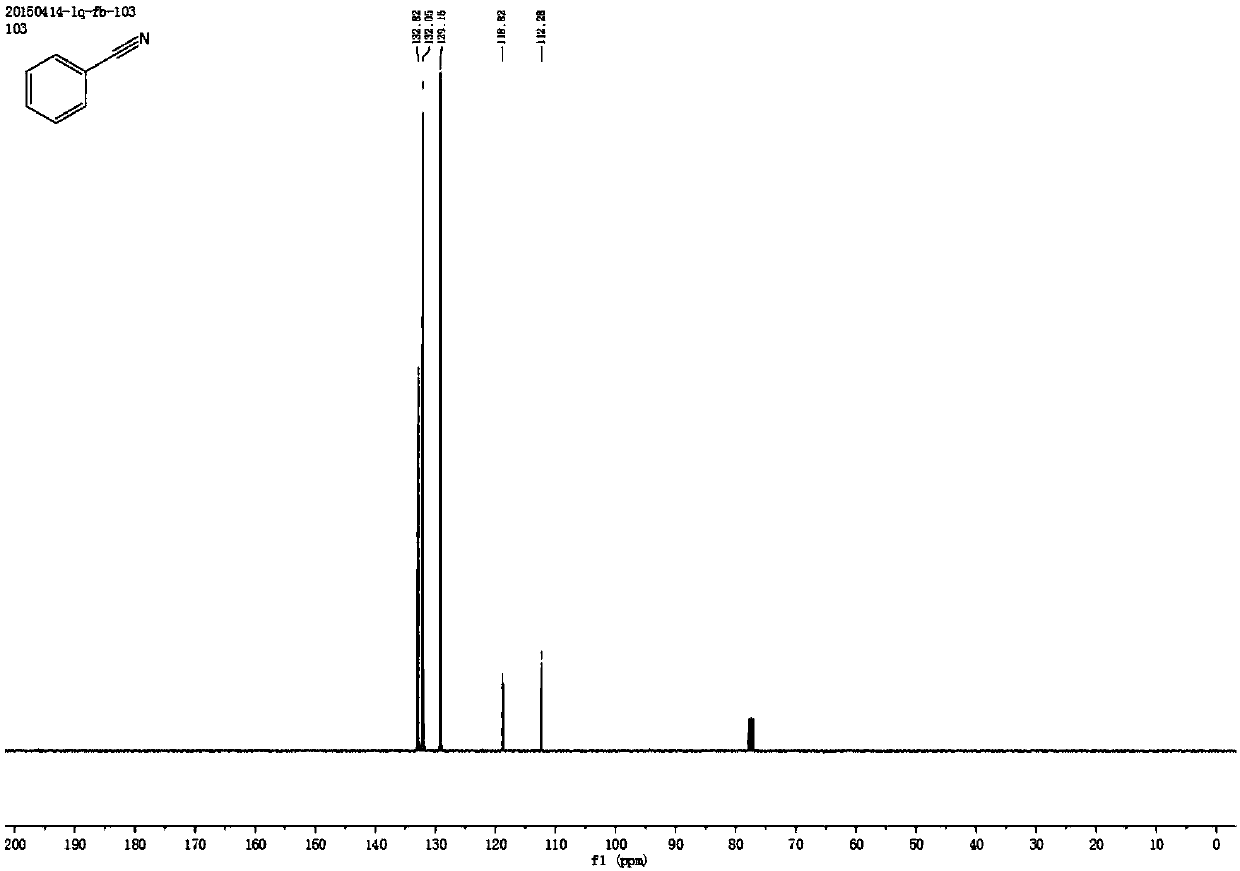
[0053] Synthesis of Benzonitrile
[0054] Add 0.4 mmol styrene, 2 mmol sodium nitrite, 1 mg metallic iron (Ⅲ) porphyrin, and 4.5 ml acetonitrile solvent to the reaction test tube, heat and stir at 70°C under air atmosphere, and add dropwise within the first 0.5 hours 0.5 milliliters of formic acid, after reacting for 4 hours, stop heating and stirring, cool to room temperature, obtain crude product through rotary evaporator, then separate and purify by column chromatography, obtain target product, the eluent of column chromatography used is petroleum ether and ethyl acetate Esters mixed solvents. The structure of benzonitrile is shown in the following formula:
[0055]
[0056] The compound is a colorless liquid with a yield of 84%, and its NMR data are as follows:
[0057] 1 H NMR (400MHz, CDCl 3 )δ7.60(dd, J=12.0,7.6Hz,3H),7.45(t,J=7.8Hz,2H); 13 CNMR (101MHz, CDCl 3 )δ132.82, 132.05, 129.16, 118.82, 112.28.
Embodiment 2
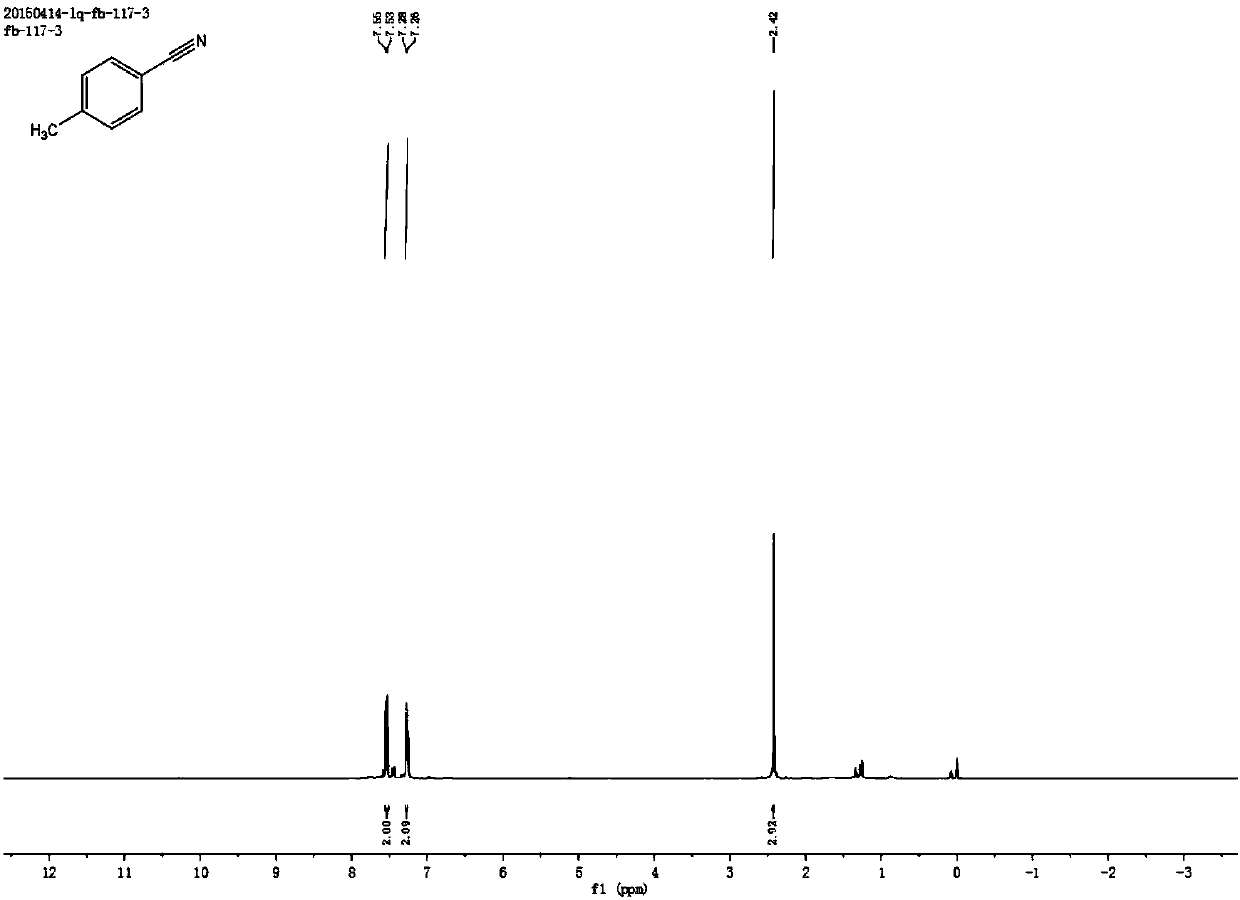
[0059] Synthesis of p-methylbenzonitrile
[0060] Add 0.4 mmol of p-methylstyrene, 2 mmol of sodium nitrite, 0.5 mg of metal iron (Ⅲ) porphyrin, and 4.5 ml of acetonitrile solvent into the reaction test tube, heat and stir at 70°C under air atmosphere, gradually within the first 0.5 hours Add 0.5 milliliters of formic acid drop by drop, after reacting for 4 hours, stop heating and stirring, cool to room temperature, obtain the crude product by rotary evaporator, then separate and purify by column chromatography to obtain the target product, the eluent of column chromatography used is petroleum ether mixed solvent with ethyl acetate. The structure of p-methylbenzonitrile is shown in the following formula:
[0061]
[0062] The compound is a colorless liquid with a yield of 87.26%, and its NMR data are as follows:
[0063] 1H NMR (400MHz, CDCl3) δ7.54 (d, J = 8.1Hz, 2H), 7.26 (d, J = 2.1Hz, 2H), 2.42 (s, 3H); 13C NMR (101MHz, CDCl3) δ 143.71, 132.06 ,129.85,119.18,109.33,2...
Embodiment 3
[0065] Synthesis of m-methylbenzonitrile
[0066] Add 0.4 mmol of m-methylstyrene, 2 mmol of sodium nitrite, 2 mg of metal iron (Ⅲ) porphyrin, and 4.5 ml of acetonitrile solvent into the reaction test tube, heat and stir at 70°C under air atmosphere, gradually within the first 0.5 hours Add 0.5 milliliters of formic acid drop by drop, after reacting for 4 hours, stop heating and stirring, cool to room temperature, obtain the crude product by rotary evaporator, then separate and purify by column chromatography to obtain the target product, the eluent of column chromatography used is petroleum ether mixed solvent with ethyl acetate. The m-methylbenzonitrile structure is shown in the following formula:
[0067]
[0068] The compound is a colorless liquid with a yield of 71.23%, and its NMR data are as follows:
[0069] 1 H NMR (400MHz, CDCl 3 )δ7.45(d, J=4.7Hz, 2H), 7.41(d, J=7.7Hz, 1H), 7.35(t, J=5.9Hz, 1H), 2.39(s, 3H); 13 C NMR (101MHz, CDCl 3 )δ139.23, 133.64, 132.50...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com