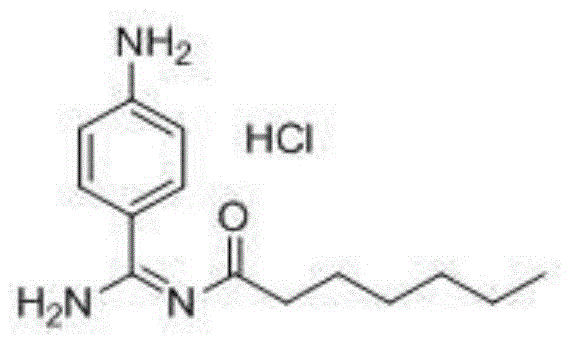
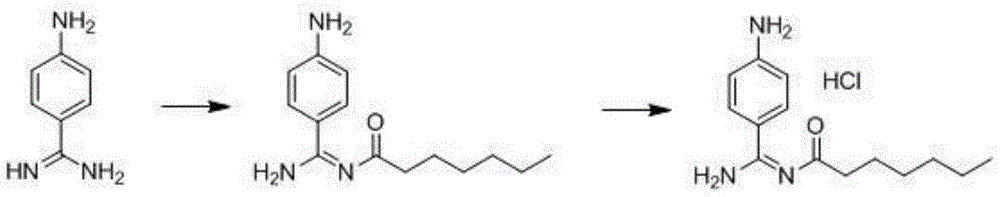
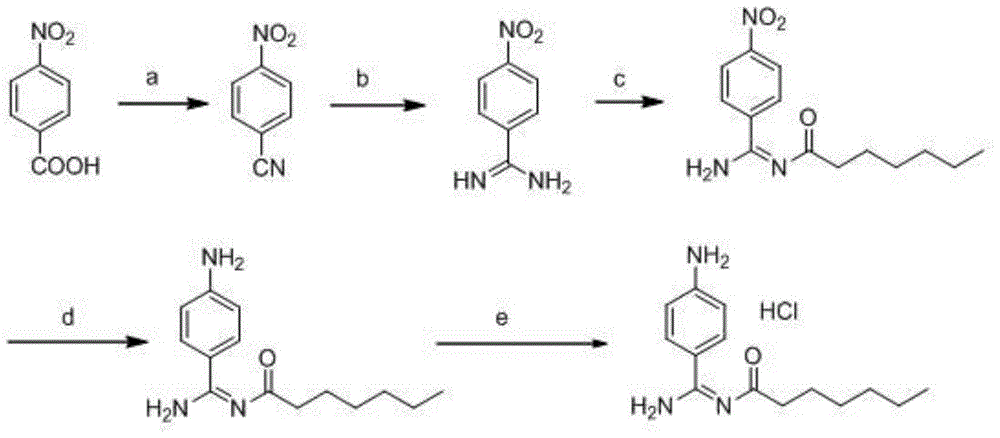
A kind of preparation method of p-aminobenzamidine hydrochloride
A technology of aminobenzamidine hydrochloride and aminobenzamidine iminoformic acid, which is applied in the field of drug synthesis, can solve the problems of many side reactions, expensive raw materials, and high cost, and achieve low price, simple reaction operation, and easy control Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] 1.1 Dissolve p-nitrobenzaldehyde (15.1 g, 0.1 mol) in N,N-dimethylacetamide, add hydroxylamine hydrochloride (8.4 g, 0.12 mol) and react at 100°C for 4 hours, after the reaction is complete, pour it into ice water, Suction filtration, washing with water, and drying gave 10.2 g of p-nitrobenzonitrile with a yield of 69%.
[0055] 1.2 Add 100mL of anhydrous methanol and 3.78g (0.07mol) of solid sodium methoxide into the reaction vessel, stir to dissolve, add 10g (0.068mol) of p-nitrobenzonitrile, and stir at 50°C for 5 hours. Then add 5.4 g (0.07 mol) of ammonium acetate, stir at 45°C for 18 hours, cool to room temperature and suction filter, rinse the filter cake with methanol, and dry in vacuo to obtain 6.6 g of p-nitrobenzamidine with a yield of 60.1%.
[0056] 1.3 Add 80mL of 30% potassium carbonate solution to the reaction vessel, stir and cool down to 0°C, add 15g (0.091mol) of p-nitrobenzamidine, 80mL of ethyl acetate, dropwise add 15g (0.091mol) of chloroformic ac...
Embodiment 2
[0062] 2.1 Dissolve p-nitrobenzaldehyde (15.1 g, 0.1 mol) in N,N-dimethylacetamide, add hydroxylamine hydrochloride (8.4 g, 0.12 mol), and ferric chloride (9.8 g, 0.06 mol) at 100°C for reaction After 3 hours, the reaction was completed, poured into ice water, filtered with suction, and dried to obtain 11.1 g of p-nitrobenzonitrile with a yield of 75%.
[0063] 2.2 Add 80mL of anhydrous methanol, stir and dissolve 4.4g (0.08mol) of solid sodium methoxide, then add 12g (0.08mol) of p-nitrobenzonitrile, stir at 30°C for 7 hours, add 6.3g (0.08mol) of ammonium chloride, 5ml ( 0.08mol) of acetic acid, stirred at 60°C for 8 hours, cooled to room temperature and suction filtered, the filter cake was rinsed with methanol, and dried in vacuo to obtain 8.4 g of p-nitrobenzamidine with a yield of 63.6%.
[0064] 2.3 Add 80mL of 4M sodium hydroxide solution, stir to cool down to 20°C, add 15g (0.091mol) of p-nitrobenzamidine, 20mL of acetone, and dropwise add 15g (0.091mol) of n-hexyl ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com