Synthesis method of ambroxol hydrochloride
A technology of ambroxol hydrochloride and a synthetic method, applied in the field of drug synthesis, can solve problems such as increased risk, many side reactions, and increased cost, and achieve the effects of simplifying production process, low product cost, and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
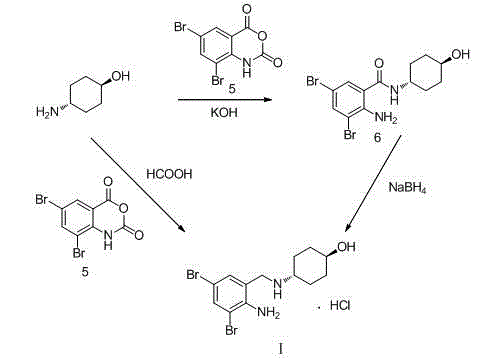
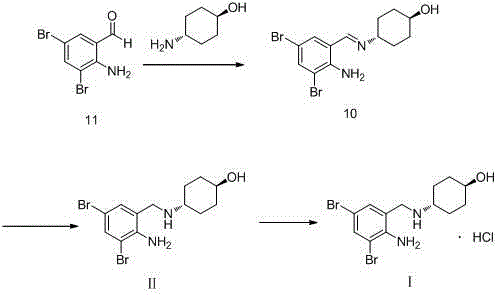
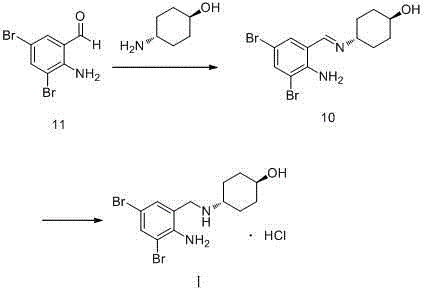
[0034] (1) Add 20g of o-nitrobenzaldehyde and 50ml of methanol into the reaction flask in turn, stir, and add 23.26g of bromine dropwise while keeping the temperature at 40°C. After the dropwise addition, continue to react at this temperature for 3h. Filtration, the filter cake was recrystallized with methanol, and dried to obtain 39.37g of compound IV, with a yield of 96.5%;
[0035] (2) Add 30.89g of compound IV obtained in step (1), 12.67g of trans-4-aminocyclohexanol and 308.9ml of toluene into the reaction flask in sequence, stir, heat up to reflux, and react for 5h. After the reaction, cool down , crystallized at 3°C for 1h, filtered, washed with toluene, and dried in vacuo at 50°C to obtain 40.16g of compound III, with a yield of 98.9%;
[0036] (3) Add 38.00g of compound III obtained in step (2), 380ml of methanol and 0.38g of catalyst Pd / C into the autoclave. After ventilation, fill with hydrogen to a pressure of 25kg, raise the temperature to 70°C, and keep stirrin...
Embodiment 2
[0039] (1) Add 20g of o-nitrobenzaldehyde and 50ml of methanol into the reaction flask in turn, stir, and add 23.26g of bromine dropwise while keeping the temperature at 50°C. After the dropwise addition, continue to react at this temperature for 3 hours. After filtration, the filter cake was recrystallized with methanol, and dried to obtain 39.41 g of compound IV, with a yield of 96.6%;
[0040] (2) Add 30.89g of compound IV obtained in step (1), 17.28g of trans-4-aminocyclohexanol and 308.9ml of toluene into the reaction flask in sequence, stir, heat up to reflux, and react for 3h. After the reaction, cool down , crystallized at 3°C for 1h, filtered, washed with toluene, and dried in vacuo at 50°C to obtain 40.20g of compound III, with a yield of 99.0%;
[0041] (3) Add 38.00g of compound III obtained in step (2), 380ml of ethanol and 0.38g of catalyst Pd / C into the autoclave. After ventilation, fill with hydrogen to a pressure of 25kg, raise the temperature to 70°C, and k...
Embodiment 3
[0044] (1) Add 20g of o-nitrobenzaldehyde and 50ml of methanol into the reaction flask in turn, stir, and add 23.26g of bromine dropwise while keeping the temperature at 20°C. After the dropwise addition, continue to react at this temperature for 3 hours. Filtration, the filter cake was recrystallized with methanol, and dried to obtain 38.57g of compound IV, with a yield of 94.6%;
[0045] (2) Add 30.89g of compound IV obtained in step (1), 14.97g of trans-4-aminocyclohexanol and 308.9ml of toluene into the reaction flask in turn, stir, heat up to reflux, and react for 8h. After the reaction is completed, cool down , crystallized at 3°C for 1 h, filtered, washed with toluene, and dried the solid under vacuum at 50°C to obtain 39.73g of compound III, with a yield of 97.8%;
[0046] (3) Add 38.00g of compound III obtained in step (2), 380ml of isopropanol and 0.38g of catalyst Pd / C into the autoclave. After ventilation, fill with hydrogen to a pressure of 25kg, raise the tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com