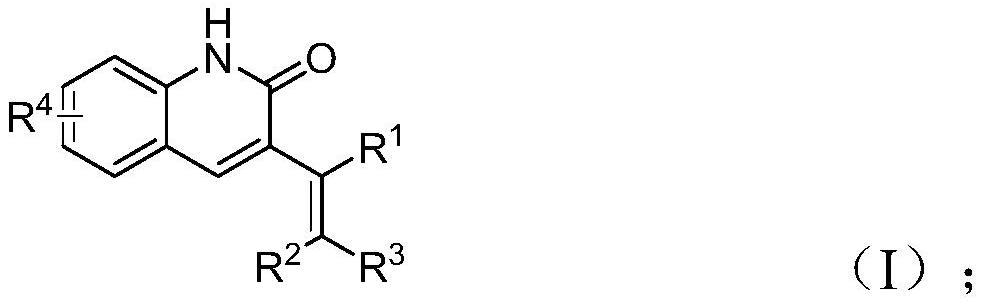
Preparation method of 3-alkenyl quinoline-2 (1H) ketone derivative
An alkenylquinoline and derivative technology, which is applied in the field of preparation of 3-alkenylquinoline-2 ketone derivatives, can solve problems such as limited research on carbonylation reaction, and achieves simple preparation method, wide tolerance range and easy operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~15
[0033] Add palladium acetate, tris(3-methoxyphenyl)phosphine, molybdenum carbonyl, cesium carbonate, tetrabutylammonium iodide, o-nitrobenzaldehyde (II) into a 15mL sealed tube according to the raw material ratio in Table 1 and allyl aryl ether (III), then add acetonitrile (1mL), mix and stir evenly, react according to the reaction conditions in Table 2, after the reaction is completed, filter, sample with silica gel, and obtain the corresponding 3- Alkenyl quinoline-2 (1H) ketone derivatives (I), the reaction process is shown in the following formula:
[0034]
[0035] The raw material addition of table 1 embodiment 1~15
[0036]
[0037] Table 2
[0038]
[0039]
[0040] In Table 1 and Table 2, T is the reaction temperature, t is the reaction time, Me is methyl, Pr is propyl, t-Bu is methyl, OMe is methoxy, CF 3 For trifluoromethyl.
[0041] The structural confirmation data of the compounds prepared in Examples 1-5:
[0042] The nuclear magnetic resonance of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com