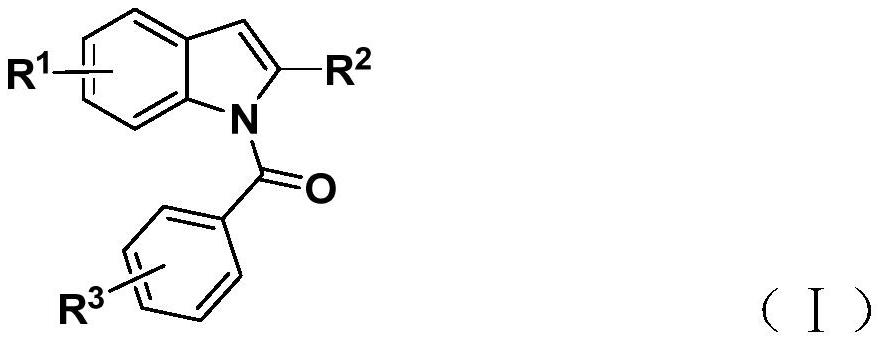
Preparation method of N-acyl indole compound
A technology of acyl indole compound and carbon monoxide, which is applied in the field of preparation of N-acyl indole compound, can solve problems such as not widely used, and achieve the effects of strong practicability, simple post-processing, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] The present invention will be further described below in conjunction with specific embodiments.
[0031] Add tetrakis(triphenylphosphine)palladium, potassium carbonate, phenol 1,3,5-tricarboxylate, 2-alkynylaniline (II), and aryl iodide into a 35mL Schlenk tube according to the raw material ratio in Table 1 (III) and 2 mL of organic solvent, mixed and stirred evenly, reacted at 60° C. for 24 hours, then added silver oxide, and continued to react at 60° C. for 24 hours, as shown in Table 1. After the reaction is complete, filter, sample with silica gel, and obtain the corresponding N-acylindole compound (I) through column chromatography purification, the reaction process is shown in the following formula:
[0032]
[0033] The raw material addition of table 1 embodiment 1~15
[0034]
[0035]
[0036] Table 2
[0037]
[0038] In Table 1 and Table 2, T is the reaction temperature, t is the reaction time, Me is methyl, tBu is tert-butyl, OMe is methoxy, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com