2,2'-dinitrodibenzyl preparation method
A technology of dinitrobibenzyl and nitrophenyl, applied in the field of preparation of intermediates, can solve problems such as increased processing cost, low product purity, explosion, etc., and achieves reduction of production cost, avoidance of high pollution, and good product purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
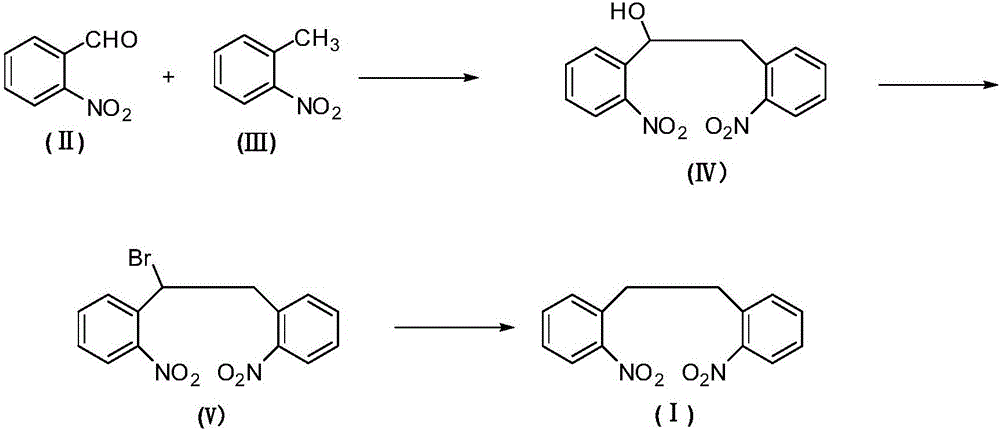
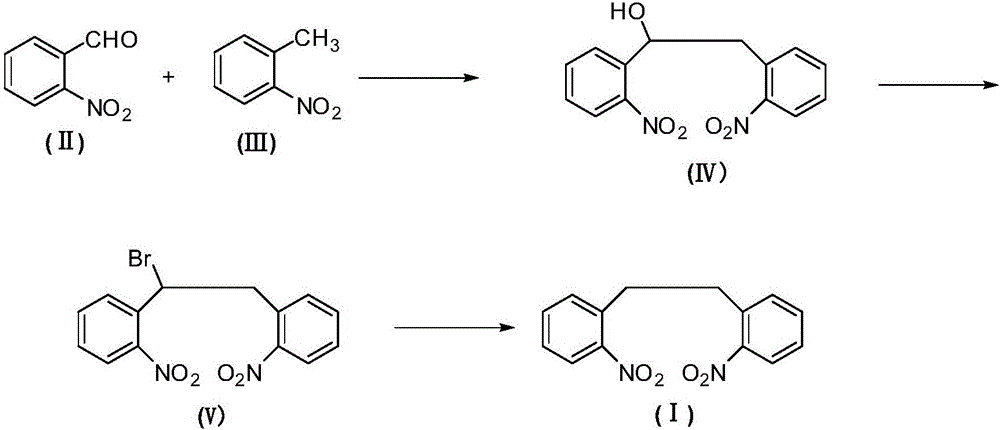
[0025] C. Preparation of 2,2'-dinitrobibenzyl (Ⅰ)
[0026] Add 1,2-bis(2-nitrophenyl)bromoethane (Ⅴ) (351g, 1.0mol) and diethylene glycol dimethyl ether (1400g) into the reactor, after stirring evenly, add boron in batches Sodium hydride (38g, 1.0mol), control the rate of addition to keep the temperature of the reaction mixture no more than 30°C during the addition process, if necessary, use a water bath to cool down, continue to stir and react at room temperature for 2 hours after the addition of sodium borohydride, after the reaction finishes, add The mixture was poured into an appropriate amount of water, and a yellow solid precipitated out, which was collected by filtration and dried to obtain crude 2,2'-dinitrobibenzyl (I), which was recrystallized from methanol (702g) to obtain a light yellow crystalline powder, 2,2 The refined product of '-dinitrobibenzyl (Ⅰ) is 251.7g, and the yield is about 92.5%.
[0027] Melting point: 120-121°C, 1 H-NMR (CDCl 3 ):δ3.09(4H),7.48-...
Embodiment 2
[0029] Other steps are the same as in Example 1, except that the preparation method of 1,2-bis(2-nitrophenyl)ethanol (IV) of A step is as follows:
[0030]In the reactor, add o-nitrotoluene (II) (137g, 1.0mol) and dimethyl sulfoxide (280g), then add potassium hydroxide (56g, 1.0mol) and ionic liquid 3-methyl-1-ethane Imidazole trifluoroacetate (13.7g), stir well after addition, cool the mixture to -5-0°C with an ice-salt bath, then add o-nitrobenzaldehyde (Ⅲ) (151g) dropwise at this temperature , 1.0mol). After the dropwise addition, keep stirring and reacting at -5-5°C for 4 hours. After the reaction, naturally rise to room temperature, pour the reaction mixture into ice water, adjust the pH of the mixture to 2-3 with 5% dilute hydrochloric acid, and continue to room temperature. After stirring for 30 minutes, the precipitated yellow solid was collected by filtration. After drying, it was 217.4 g of 1,2-bis(2-nitrophenyl)ethanol (IV), with a yield of about 75.5%.
Embodiment 3
[0032] Other steps are the same as in Example 1, except that the preparation method of 1,2-bis(2-nitrophenyl)ethanol (IV) of A step is as follows:
[0033] In the reactor, add o-nitrotoluene (II) (137g, 1.0mol) and dimethyl sulfoxide (1370g), then add potassium hydroxide (58.8g, 1.05mol) and ionic liquid 3-methyl-1- Ethyl imidazole trifluoroacetate (13.7g), stir evenly after adding, cool the mixture to -5-0°C with an ice-salt bath, then add o-nitrobenzaldehyde (Ⅲ) dropwise at this temperature ( 151 g, 1.0 mol). After the dropwise addition, keep stirring and reacting at -5-5°C for 5 hours. After the reaction, naturally rise to room temperature, pour the reaction mixture into ice water, adjust the pH of the mixture to 2-3 with 5% dilute hydrochloric acid, and continue to room temperature. After stirring for 30 minutes, the precipitated yellow solid was collected by filtration, and after drying, it was 220.1 g of 1,2-bis(2-nitrophenyl)ethanol (IV), with a yield of about 76.4%. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com