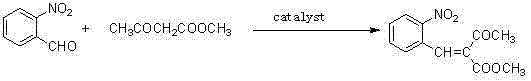
Preparation method of methyl 2-nitrobenzal acetoacetate
A technology of methyl nitrobenzylidene acetoacetate and methyl acetoacetate is applied in the field of preparation of methyl 2-nitrobenzylidene acetoacetate, can solve problems such as residues, cannot be fully reacted, and achieves short reaction time , the effect of high purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The chemical reagents and chemicals in the examples of the present invention are all analytically pure.
[0028] The preparation method of 2-nitrobenzylidene acetoacetate methyl ester of the present invention comprises the following steps:
[0029] (1) In a 250ml three-necked flask, add 15.1g of o-nitrobenzaldehyde, 13.92g of methyl acetoacetate, 25ml of organic solvent methanol, and 0.44g of catalyst piperidine acetate, and react at 70°C for 2 hours to generate 2-nitrobenzyl Methyl acetoacetate was concentrated in vacuo to remove methanol to obtain a crude reddish-brown oily product of methyl 2-nitrobenzylidene acetoacetate.
[0030] Concrete reaction route is as follows:
[0031]
[0032] (2) Add 50ml of ethyl acetate / petroleum ether (volume ratio 1:5) mixture to the crude product of reddish-brown 2-nitrobenzylidene acetoacetate oil, stir at 0°C for 2h and filter to obtain About 25.8 g of solid crude methyl 2-nitrobenzylidene acetoacetate.
[0033] (3) The solid...
Embodiment 2
[0035] (1) In a 250ml three-necked flask, add 15.1g of o-nitrobenzaldehyde, 13.92g of methyl acetoacetate, 25ml of ethanol, and 0.41g of pyridinium acetate. After reacting at 80°C for 2 hours, concentrate in vacuo to remove the ethanol to obtain reddish-brown 2 - Methyl nitrobenzylidene acetoacetate oil.
[0036] (2) Add 50ml of ethyl acetate / cyclohexane (volume ratio: 1:5) mixture to 2-nitrobenzylidene acetoacetate, stir at 0°C for 2h and filter to obtain about 25.1g of wet product ,
[0037] (3) The solid crude product of methyl 2-nitrobenzylidene acetoacetate was recrystallized with 75ml of methanol to obtain a white solid, about 21.4g after drying, with a yield of about 85.9% and a purity of more than 99.5%.
Embodiment 3
[0039] (1) In a 250ml three-neck flask, add 15.1g of o-nitrobenzaldehyde, 13.92g of methyl acetoacetate, 25ml of isopropanol, and 0.38g of pyridinium formate. After reacting at 80°C for 2 hours, concentrate in vacuo to remove ethanol to obtain red Brown 2-nitrobenzylidene acetoacetate methyl ester oil.
[0040] (2) Add 50ml of ethyl acetate / cyclohexane (volume ratio: 1:5) mixture to 2-nitrobenzylidene acetoacetate, stir at 0°C for 2h and filter to obtain about 25.1g of wet product ,
[0041] (3) The solid crude product of methyl 2-nitrobenzylidene acetoacetate was recrystallized with 75ml of methanol to obtain a white solid, about 21.4g after drying, with a yield of about 85.9% and a purity of more than 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com