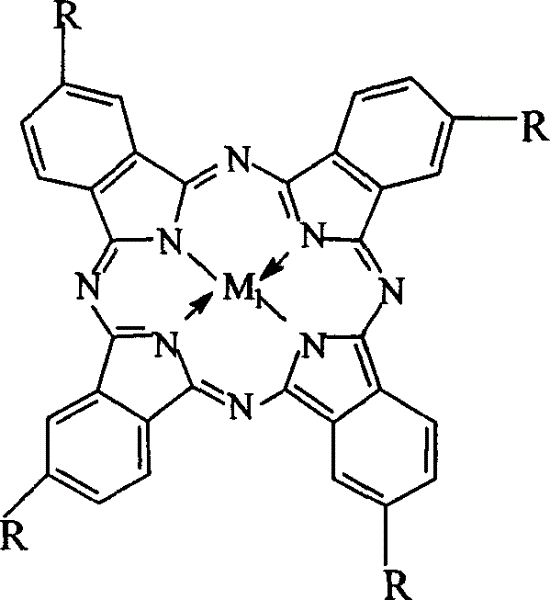
Preparation of o-nitrobenzaldehyde by biomimetic catalysis oxidation of o-nitrotoluene with oxygen
A technology for o-nitrobenzaldehyde and o-nitrotoluene, which is applied in the field of biomimetic catalytic oxygen oxidation of o-nitrotoluene to prepare o-nitrobenzaldehyde, can solve the problems of high equipment cost, large equipment corrosion, complicated operation and the like, and achieves a reaction Low temperature, reduced reaction cost and small dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Get 7mg cobalt phthalocyanine (i.e. R=H in general formula (I), M 1 =Co), 3.5g of o-nitrotoluene and 5.6g of sodium hydroxide were charged into a 200ml autoclave, 30ml of methanol was added, and oxygen at a pressure of 2.5MPa was introduced, and the reaction was carried out in a water bath at a temperature of 40°C for 12h. The mixed solution after the reaction removes the catalyzer first through suction filtration, then adds 30ml distilled water, adds dilute hydrochloric acid to neutralize, filters, and uses high-pressure liquid chromatography to analyze and detect after purification, and the yield of o-nitrobenzaldehyde is 15.6%. The product purity is 98.5%.
Embodiment 2
[0025] Get 15mg iron phthalocyanine (i.e. R=H in general formula (I), M 1 =Fe), 2.5g of o-nitrotoluene and 3.6g of sodium hydroxide were put into an autoclave, 30ml of methanol was added, the oxygen pressure was 3.0MPa, the temperature was controlled at 40°C in a water bath, and the reaction was carried out for 18h. The processing steps are the same as Example 1, and the product obtained is analyzed and detected by high-pressure liquid chromatography, and the yield of o-nitrobenzaldehyde is 13.5%, and the product purity after purification is 99.0%.
Embodiment 3
[0027] Get 14mg copper phthalocyanine (being R=H in the general formula (I), M 1 =Cu), 3.5g of o-nitrotoluene and 6.4g of sodium hydroxide were put into an autoclave, 30ml of methanol was added, the oxygen pressure was 1.0MPa, the temperature was controlled at 25°C in a water bath, and the reaction was carried out for 48h. The processing steps are the same as in Example 1, and the resulting product is analyzed and detected by high-pressure liquid chromatography. The yield of o-nitrobenzaldehyde is 18.4%, and the purified product has a purity of 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com