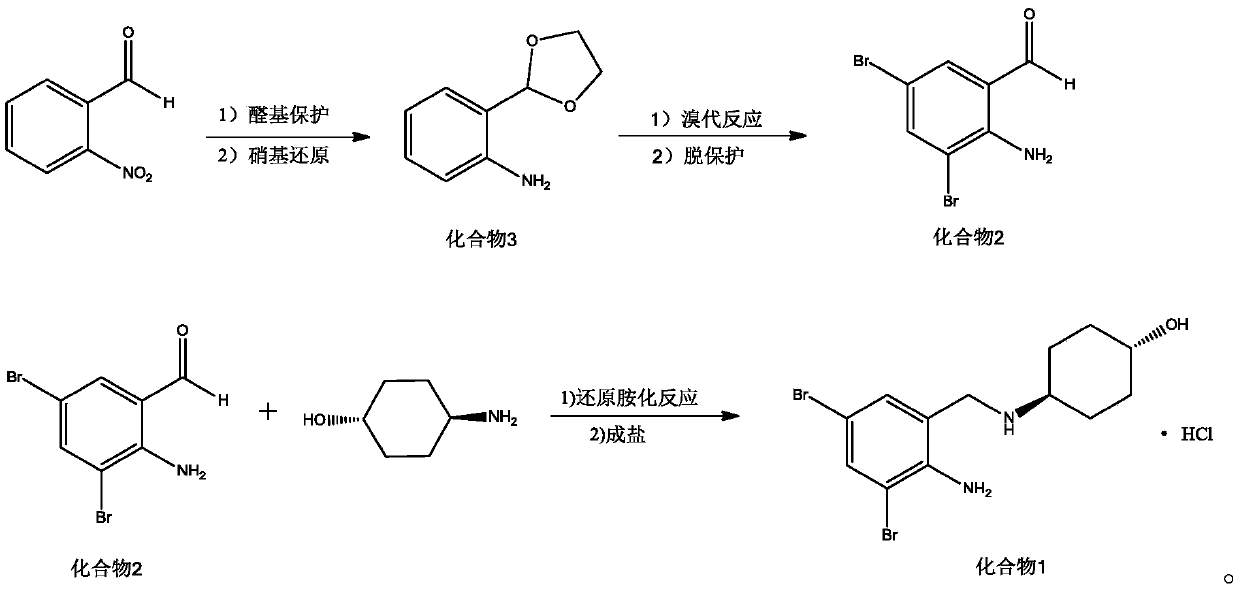
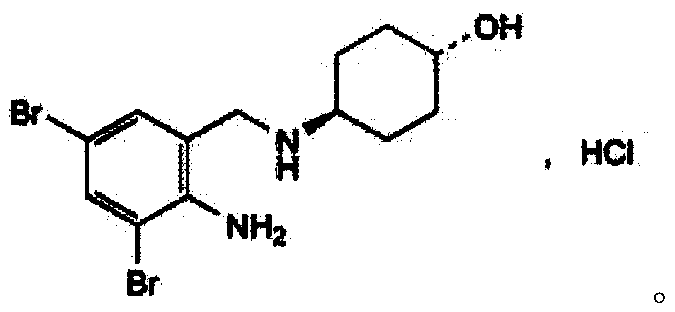
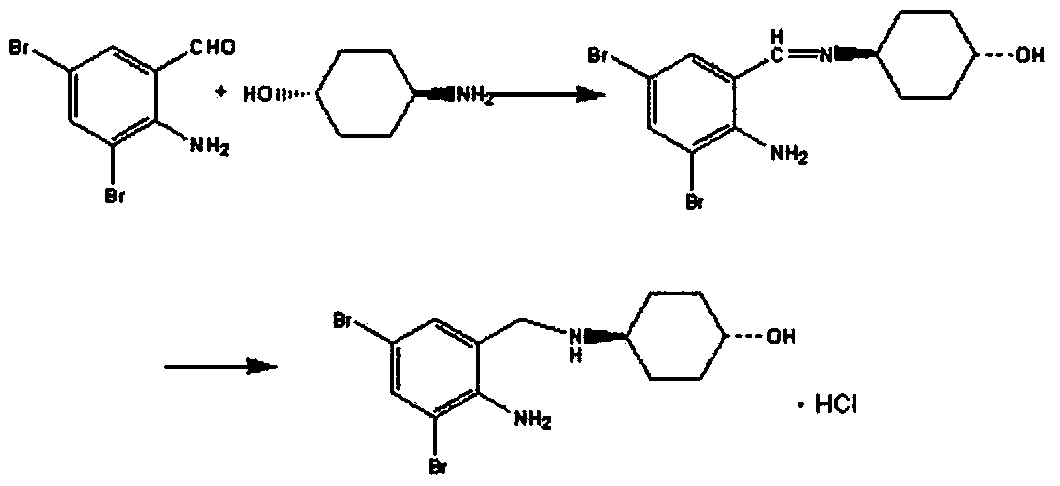
Preparation method of ambroxol hydrochloride
A technology of ambroxol hydrochloride and salt formation, which is applied in the field of drug synthesis, can solve the problems of industrial equipment corrosion, high risk, and increased risk, and achieve the effects of reduced service life, simple reaction steps, and improved utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the synthesis of compound 3
[0035] Dissolve 50mmol (7.556g) of o-nitrobenzaldehyde, 60mmol of ethylene glycol, and 0.25mmol of p-toluenesulfonic acid monohydrate in 100mL of toluene, heat to reflux for 1.5h, and separate water with a Dean-Stark separator. After the reaction, the solution Rotary evaporate, add 100mL of ethyl acetate to dissolve, wash with 50mL of sodium bicarbonate aqueous solution twice, and dry to obtain the crude product. Dissolve the crude product in 100mL THF, then add the catalyst 1g NiCoB / TiO 2 , 7g of hydrazine hydrate, under the protection of nitrogen, start stirring, raise the temperature to 50-55°C, react for 90min, and monitor the reaction progress by TLC. After the reaction, the catalyst was filtered, the solvent was removed by rotary evaporation, 50 mL of diethyl ether was added to stir and crystallize for 20 min, and 7.815 g of compound 3 was obtained after suction filtration and drying, with a yield of 94.50% and a purity...
Embodiment 2
[0036] Embodiment 2: the synthesis of compound 3
[0037] Dissolve 50mmol (7.556g) of o-nitrobenzaldehyde, 50mmol of ethylene glycol, and 0.1mmol of p-toluenesulfonic acid monohydrate in 100mL of toluene, heat to reflux for 1.5h, and separate water with a Dean-Stark separator. After the reaction is complete, the solution Rotary evaporate, add 100mL of ethyl acetate to dissolve, wash with 50mL of sodium bicarbonate aqueous solution twice, and dry to obtain the crude product. Dissolve the crude product in 100mL THF, then add the catalyst 1g NiCoB / TiO 2 , 7g of hydrazine hydrate, under the protection of nitrogen, start stirring, raise the temperature to 50-55°C, react for 90min, and monitor the reaction progress by TLC. After the reaction, the catalyst was filtered, the solvent was removed by rotary evaporation, 50 mL of diethyl ether was added to stir and crystallize for 20 min, and 7.339 g of compound 3 was obtained after suction filtration and drying, with a yield of 88.24% a...
Embodiment 3
[0038] Embodiment 3: the synthesis of compound 3
[0039] Dissolve 50mmol (7.556g) of o-nitrobenzaldehyde, 70mmol of ethylene glycol, and 0.4mmol of p-toluenesulfonic acid monohydrate in 100mL of toluene, heat to reflux for 1.5h, and separate the water with a Dean-Stark separator. After the reaction, the solution Rotary evaporate, add 100mL of ethyl acetate to dissolve, wash with 50mL of sodium bicarbonate aqueous solution twice, and dry to obtain the crude product. Dissolve the crude product in 100mL THF, then add the catalyst 1g NiCoB / TiO 2 , 7g of hydrazine hydrate, under the protection of nitrogen, start stirring, raise the temperature to 50-55°C, react for 90min, and monitor the reaction progress by TLC. After the reaction, the catalyst was filtered, the solvent was removed by rotary evaporation, 50 mL of diethyl ether was added to stir and crystallize for 20 min, and 7.352 g of compound 3 was obtained after suction filtration and drying, with a yield of 89.36% and a pur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com