Levamlodipine compound prepared in novel method
A technology of levamlodipine besylate and levamlodipine, which is applied in the field of medicine, can solve the problems of difficult recovery of solvents, disproportionation reaction, and difficult to recover, and achieves less environmental pollution, less skin irritation, and lower production costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
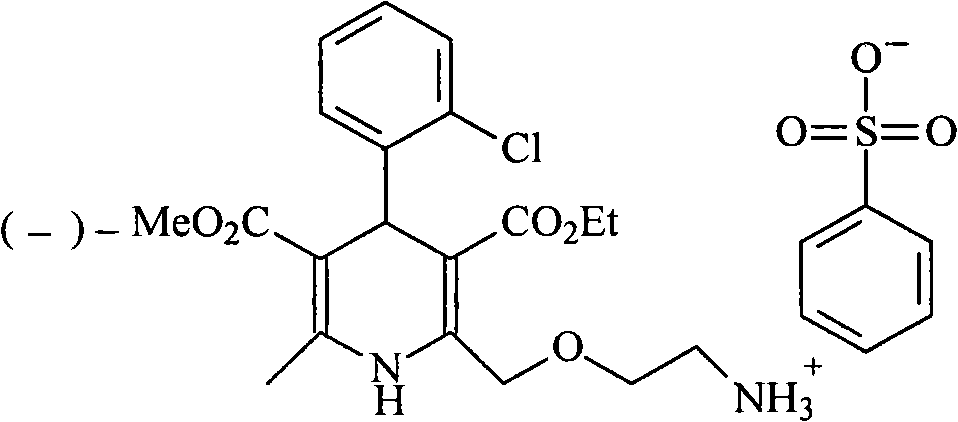
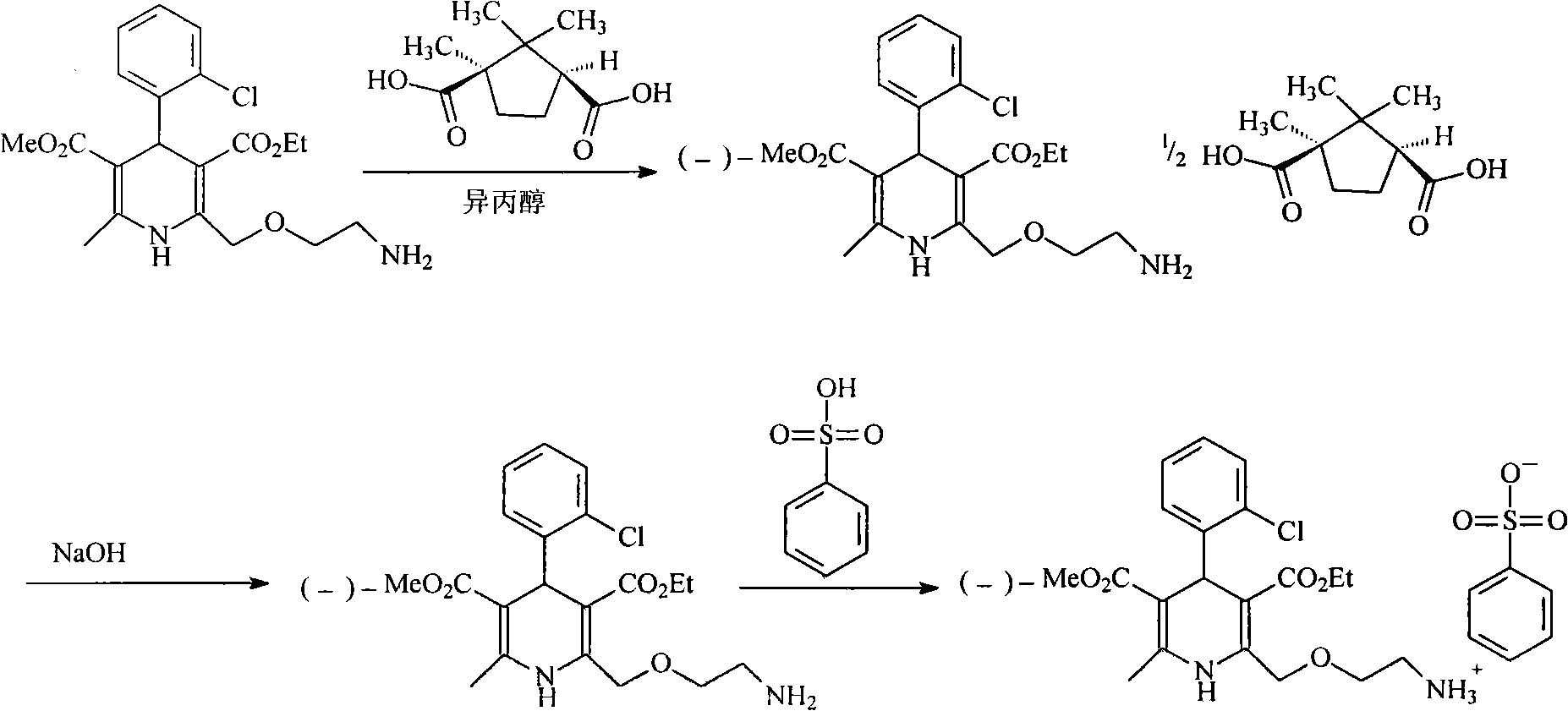
[0027] The synthesis of embodiment 1 levamlodipine besylate
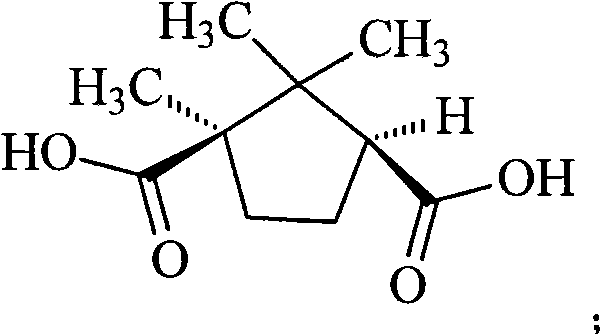
[0028] (1) Synthesis of D-(+)-Camphoric Acid Levoamlodipine
[0029] Add amlodipine base 409g (1mol) in the there-necked flask, be dissolved in 2.5L isopropanol, add the D-(+)-camphoric acid (0.5mol) of 100g in batches and be dissolved in the isopropanol solution of 500ml, Stir and react at room temperature for half an hour, a large amount of solids precipitated, continue to stir and react for 12 hours, filter, add 1L of absolute ethanol to reflux the filtered crude product for half an hour, cool, precipitate solids, filter, and vacuum dry at 40°C to obtain 204g of the product , yield 80%.
[0030] (2) Synthesis of Levoamlodipine
[0031] D-(+)-Camphoric Acid Levoamlodipine 128g was added to a mixed solution of 800ml of methylene chloride and 150ml of 2mol / L aqueous sodium hydroxide solution, stirred and reacted for 1 hour, left to stand for half an hour, and the organic layer was separated. Washed twice with 200...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com