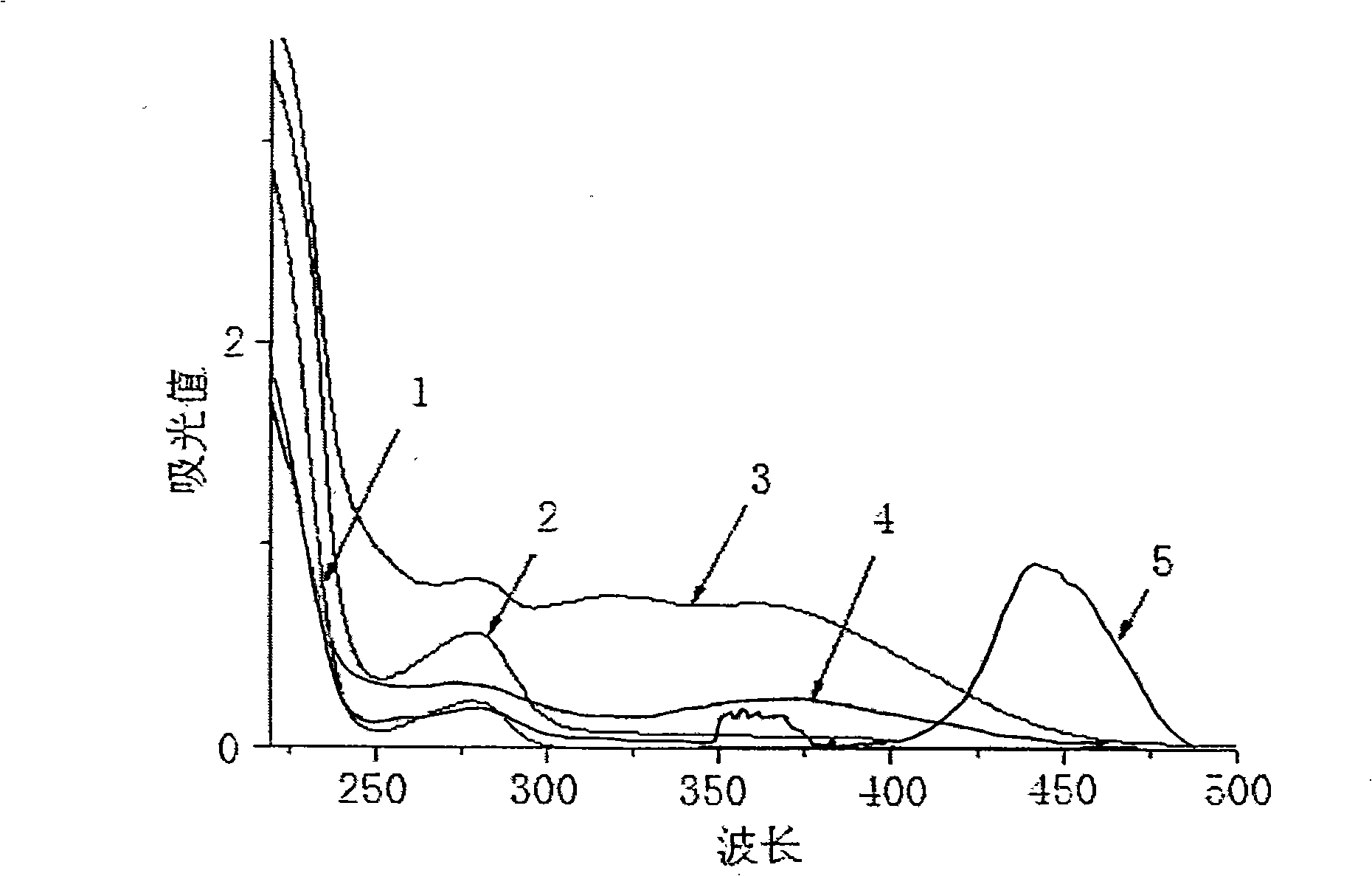
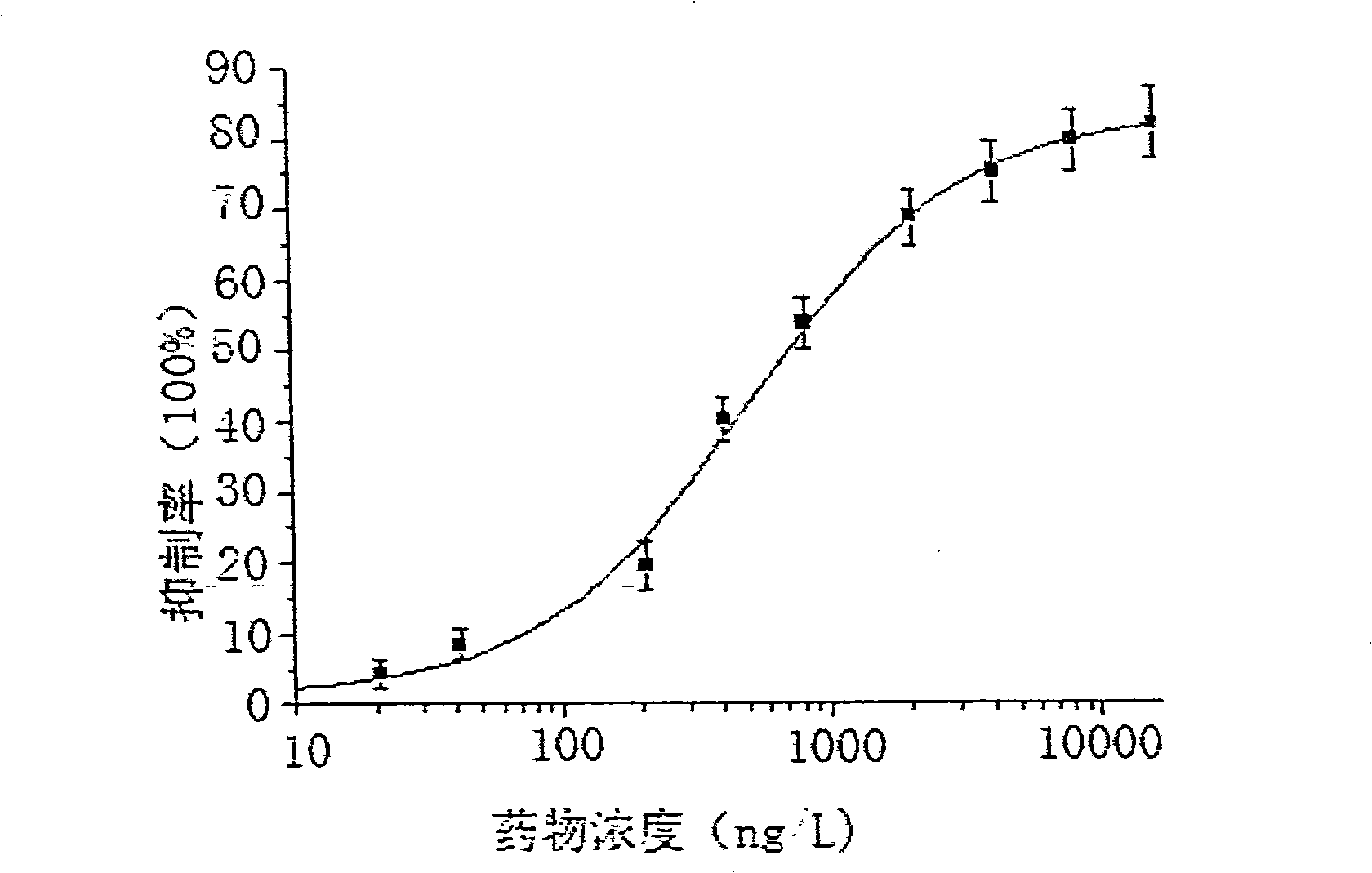
3,5-dinitrosalicylic acid hydrazine enzyme-linked immunologic detecting kit and its use method
A technology of dinitrosalicylic acid and enzyme-linked immunoassay, which is applied in the field of immunochemistry, can solve the problems of high instrument cost, time-consuming and laborious processing, and complicated processing, and achieve simplified operation, improved specificity and sensitivity, and simple pre-treatment Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The synthesis of embodiment 1 hapten
[0027] Add 10mmol of m-carboxybenzaldehyde into a 50ml round bottom flask, slowly add methanol until the m-carboxybenzaldehyde is completely dissolved, add 8mmol of 3,5-dinitrosalicylic acid hydrazine while stirring, and stir at room temperature overnight; after the reaction, filter, 20mL The precipitate was washed twice with methanol, dried, and the yellow powdery solid was CPDNSH: APCI-MS (negative) m / z: 374 [M-H] - . 1 HNMR (600MHz, d 6 -DMSO, TMS): δ14.05(s, 1H); 8.82(d, J=3.0Hz, 1H); 8.58(d, J=3.0Hz, 1H); 8.48(s, 1H); 8.35(s, 1H); 7.97(t, J=7.8Hz, 1H); 7.59(t, J=7.7Hz, 1H).
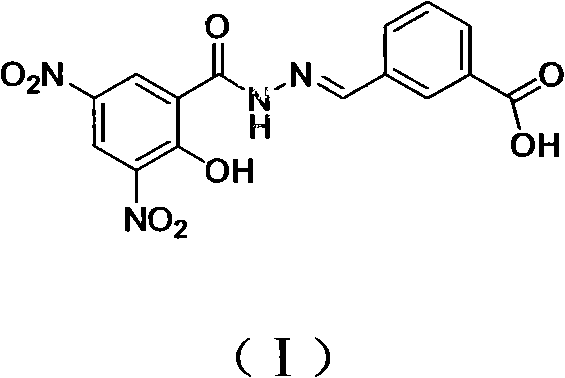
[0028] Its structure is shown in formula (I):
[0029]
Embodiment 2
[0030] Synthesis of Example 2 Antigen
[0031] Dissolve 0.1mmol of the hapten CPDNSH in 2 mL of DMF, stir and add DCC 27.5 mg and NHS 14.4 mg, and react overnight with magnetic stirring at 4°C. After centrifugation, the supernatant is liquid A; weigh 140 mg of BSA and dissolve in 10 mL at a concentration of 0.1 mol / Add 1 mL of DMF to PBS (pH 8.0) in L, stir and dissolve to prepare liquid B; under magnetic stirring, liquid A is gradually dropped into liquid B, and react at 4°C for 12 hours; after centrifugation, take the supernatant and use physiological Saline dialysis was performed for 3 days, and the dialysate was changed 3 times a day to obtain the whole antigen CPDNSH-BSA. Packed at a concentration of 1g / L. Freeze and store in -20°C refrigerator for immunization. The structure of CPDNSH-BSA is shown in formula (II):
[0032]
Embodiment 3
[0033] The synthesis of embodiment 3 coating original
[0034] Dissolve 0.1mmol of the hapten CPDNSH in 2mL of DMF, stir and add 27.5mg of DCC and 14.4mg of NHS, and react overnight with magnetic stirring at 4°C. After centrifugation, the supernatant is A liquid; Add 1 mL of DMF to 0.1mol / L PBS (PH8.0), stir and dissolve to prepare solution B; under magnetic stirring, gradually drop solution A into solution B, and react at 4°C for 12 hours; after centrifugation, take the supernatant for 4 nights. Dialyze with normal saline for 3 days at ℃, change the dialysate 3 times a day, and obtain the coated original CPDNSH-OVA. Packed at a concentration of 1g / L. Store frozen in a -20°C refrigerator for wrapping.
[0035] The coating former CPDNSH-OVA of preparation has structure shown in formula (III):
[0036]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com