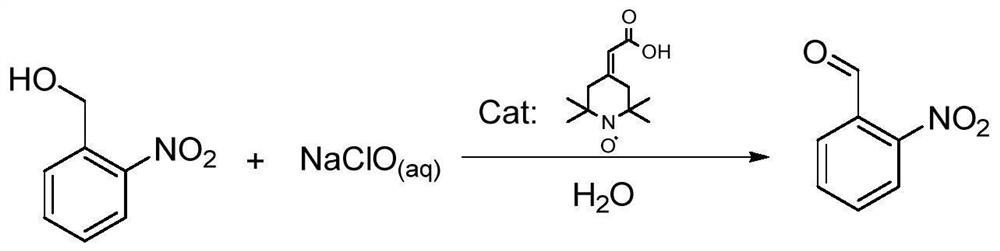
Preparation method of o-nitrobenzaldehyde
A technology of o-nitrobenzaldehyde and o-nitrobenzyl alcohol, which is applied in the field of o-nitrobenzaldehyde preparation, can solve the problems of high waste liquid treatment costs, unavoidable by-products of o-nitrobenzoic acid, and too strong oxidation conditions, etc. problems, to achieve the effect of convenient environmental protection treatment, short preparation process time, mild and moderate oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In the 2000ml reaction flask, add 153g of o-nitrobenzyl alcohol, 1.5g of 2-(2,2,6,6-tetramethylpiperidinyl nitroxide radical-4-ylidene) acetic acid, 300g of dichloromethane 1.5g of potassium bromide was dissolved in 300g of water and added to the reaction system, cooled to 5°C with an ice bath, and 84g of NaHCO 3 Dissolve it in 1000g of 10% sodium hypochlorite solution, adjust its pH to about 8.5, slowly add it to the reaction bottle, control the dropping speed so that the reaction temperature is not higher than 10°C, after the dropping is completed, TLC detects that the reaction is complete in 2 hours, and the reaction The liquid was allowed to stand for liquid separation, the aqueous phase was extracted with 500gx2 dichloromethane, the organic phase was combined, washed once with 500g of water, and the organic phase was washed with anhydrous Na 2 SO 4 Dry, filter, concentrate the light yellow oil, add 300g of methyl tert-butyl ether, stir evenly, stand at 0°C for cry...
Embodiment 2
[0031] Add 7.65 g of o-nitrobenzyl alcohol, 7.5 mg of 2-(2,2,6,6-tetramethylpiperidinyl nitroxide radical-4-ylidene) acetic acid, 15 g of dichlorobenzyl alcohol in a 100 ml reaction flask Methane and 7.5mg of sodium bromide were dissolved in 15g of water and added to the reaction system, cooled to 5°C with an ice bath, and 4.2g of NaHCO 3 Dissolve in 65g of 10% sodium hypochlorite solution, adjust its pH to about 8.5, slowly add it to the reaction bottle, control the dropping speed so that the reaction temperature is not higher than 20°C, after the dropping is completed, TLC detects that the reaction is complete in 0.5 hours, and the reaction The liquid was allowed to stand for liquid separation, the aqueous phase was extracted with 25gx2 dichloromethane, the organic phase was combined, washed once with 25g of water, and the organic phase was washed with anhydrous Na 2 SO 4 Dry, filter, and concentrate the obtained light yellow oil, add 15g of petroleum ether, stir evenly, st...
Embodiment 3
[0033] Add 7.65 g of o-nitrobenzyl alcohol, 0.8 mg of 2-(2,2,6,6-tetramethylpiperidinyl nitroxide radical-4-ylidene) acetic acid, 15 g of dichlorobenzyl alcohol into a 100 ml reaction flask Methane and 7.5mg of potassium bromide were dissolved in 15g of water and added to the reaction system, cooled to 5°C with an ice bath, and 4.2g of NaHCO 3 Dissolve in 65g of 10% sodium hypochlorite solution, adjust its pH to about 8.5, slowly add it to the reaction bottle, control the dropping speed so that the reaction temperature is not higher than 20°C, after the dropping is completed, TLC detects that the reaction is complete in 0.5 hours, and the reaction The liquid was allowed to stand for liquid separation, the aqueous phase was extracted with 25gx2 dichloromethane, the organic phase was combined, washed once with 25g of water, and the organic phase was washed with anhydrous Na 2 SO 4 Dry, filter, concentrate the light yellow oil, add 15g of petroleum ether, stir evenly, stand at 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com