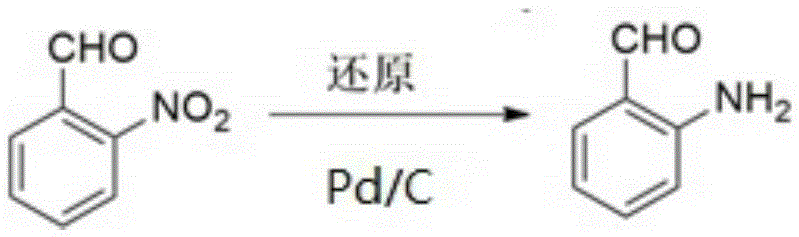
Method for synthesizing o-aminobenzaldehyde
A technology for o-aminobenzaldehyde and o-nitrobenzaldehyde is applied in the field of synthesizing o-aminobenzaldehyde and pharmaceutical intermediates, and can solve the problems of low conversion rate, low efficiency of reducing agent use, etc., and achieve high conversion rate and high efficiency. selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In the first step, 30mg of 5wt% Pd / C catalyst, 0.9g of o-nitrobenzaldehyde, 40mL of methanol and 5mL of water were added into the autoclave.
[0022] In the second step, in the autoclave, with N 2 Replace 3 times, pass H 2 , to H 2 The partial pressure is 1.5 MPa, the reaction temperature is 70° C., and the reaction time is 5 hours, so that o-nitrobenzaldehyde is converted into anthranilaldehyde.
[0023] The third step is to cool the reactor to room temperature, filter out the catalyst, wash the separated catalyst with absolute ethanol, soak it in absolute ethanol after washing, and prepare for repeated use; then extract the filtrate with dichloromethane for 3-4 times , combined the organic layers, dried with anhydrous sodium sulfate, filtered, and the filtrate was rotary evaporated to remove dichloromethane to obtain the anthranilaldehyde product.
[0024] According to high-performance liquid chromatography analysis, the conversion rate of o-nitrobenzaldehyde is 90...
Embodiment 2
[0026] In the first step, 30mg of 5wt% Pd / C catalyst, 0.9g of o-nitrobenzaldehyde, 40mL of ethanol, and 5mL of water were added into the autoclave.
[0027] In the second step, in the autoclave, with N 2 Replace 3 times, pass H 2 , to H 2 The partial pressure is 2.0 MPa, the reaction temperature is 80° C., and the reaction time is 6 hours, so that o-nitrobenzaldehyde is converted into anthranilaldehyde.
[0028] The third step is to cool the reactor to room temperature, filter out the catalyst, wash the separated catalyst with absolute ethanol, soak it in absolute ethanol after washing, and prepare for repeated use; then extract the filtrate with dichloromethane for 3-4 times , combined the organic layers, dried with anhydrous sodium sulfate, filtered, and the filtrate was rotary evaporated to remove dichloromethane to obtain the anthranilaldehyde product.
[0029] According to high-performance liquid chromatography analysis, the conversion rate of o-nitrobenzaldehyde is 90...
Embodiment 3
[0031] In the first step, 30mg of 5wt% Pd / C catalyst, 0.9g of o-nitrobenzaldehyde, 40mL of n-propanol, and 5mL of water were added into the autoclave.
[0032] In the second step, in the autoclave, with N 2 Replace 3 times, pass H 2 , to H 2 The partial pressure is 2.0 MPa, the reaction temperature is 90° C., and the reaction time is 8 hours, so that o-nitrobenzaldehyde is converted into anthranilaldehyde.
[0033] The third step is to cool the reactor to room temperature, filter out the catalyst, wash the separated catalyst with absolute ethanol, soak it in absolute ethanol after washing, and prepare for repeated use; then extract the filtrate with dichloromethane for 3-4 times , combined the organic layers, dried with anhydrous sodium sulfate, filtered, and the filtrate was rotary evaporated to remove dichloromethane to obtain the anthranilaldehyde product.
[0034] According to high-performance liquid chromatography analysis, the conversion rate of o-nitrobenzaldehyde is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com