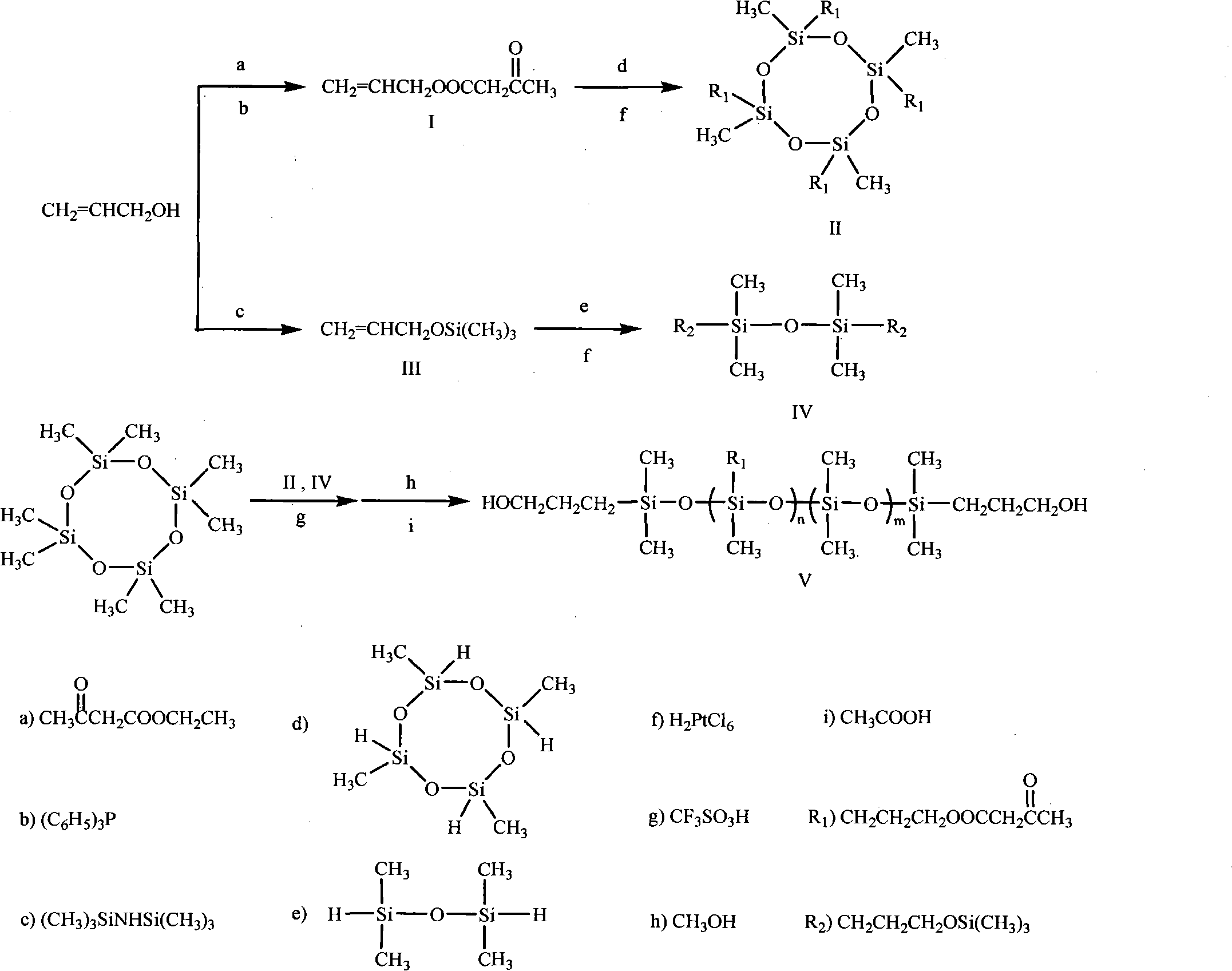
Method for synthesizing ketocarbonyl-containing bis-hydroxypropyl terminated polysiloxane
A bishydroxypropyl sealing and polysiloxane technology, applied in the direction of polyurea/polyurethane coatings, coatings, etc., can solve the problems affecting the physical and mechanical properties of the coating film, poor mechanical properties, etc., to improve the physical and mechanical properties, improve Water resistance, the effect of improving the relative molecular mass
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Transesterification reaction (molar ratio triphenylphosphine: ethyl acetoacetate: allyl alcohol = 0.1:1:1.3)
[0028] In a 100mL three-necked flask, add 13.03g of ethyl acetoacetate, 7.54g of allyl alcohol, 2.62g of triphenylphosphine and 20mL of toluene, raise the temperature of the reaction system to 100°C, and continue the reaction at this temperature for 8 hours, stop reaction. The reaction solution was distilled under reduced pressure, and the fraction at 77°C / 1.13KPa was collected to obtain 10.82 g of allyl acetoacetate, with a yield of 76%.
[0029] Hydroxyl protection reaction (molar ratio of allyl alcohol: hexamethyldisilazane == 2: 1.1)
[0030] In a 100mL three-necked flask, slowly add 35.52g of hexamethyldisilazane to 23.23g of allyl alcohol dropwise at room temperature. After the dropwise addition, raise the temperature of the reaction system to 100°C and continue the reaction at this temperature After 6 hours, the reaction stopped. Atmospheric pressure ...
Embodiment 2
[0041] Ethyl acetoacetate is changed to methyl acetoacetate in the transesterification reaction of embodiment 1, and the ratio of the amount of methyl acetoacetate and allyl alcohol becomes 1: 1, and other reaction conditions are as described in embodiment 1, obtain Allyl acetoacetate, the yield was 72%.
Embodiment 3
[0043] The ratio of the amount of allyl alcohol and hexamethyldisilazane in the hydroxyl protection reaction of Example 1 is changed to 2: 1, and other reaction conditions are as described in Example 1 to obtain allyloxytrimethylsilane, The yield was 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com