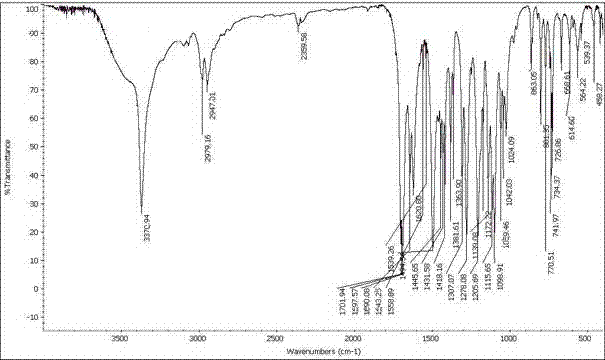
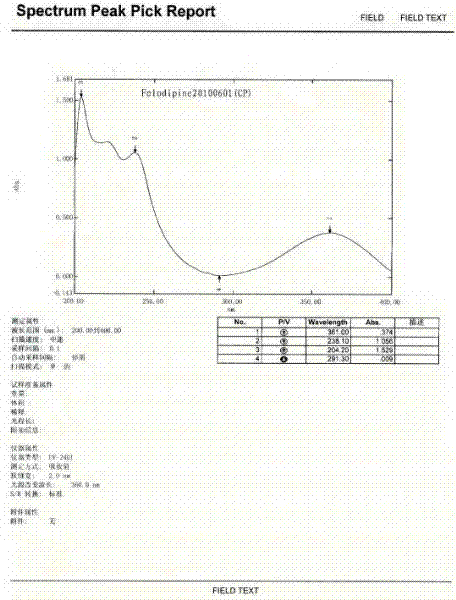
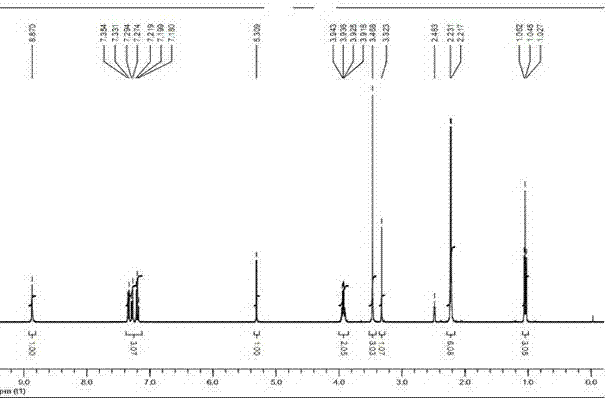
The preparation method of felodipine
A technology of felodipine and felodipine, which is applied in the field of medicine, can solve the problems of cumbersome synthetic routes, high operation requirements, and low total yield, and achieve the goals of short and effective preparation route steps, high toxicity residues, and increased drug content Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1: Preparation of 2,3-dichlorobenzylidene acetoacetate methyl ester.
[0059] Add 108ml of methyl tert-butyl ether to a 500ml reaction flask, add 20g (0.11mol) 2,3-dichlorobenzaldehyde (general formula II), 0.65g piperidine, 0.5g glacial acetic acid, 20g under stirring at room temperature (0.17mol) Methyl acetoacetate (Formula III). Increase the temperature and heat to 60-62°C to reflux, and start water separation. After 10 hours of timing reaction from the reflux, the heating was stopped and the temperature was cooled to room temperature. Wash with water to pH 7, after drying with anhydrous sodium sulfate, distill the filtrate under reduced pressure to remove methyl tert-butyl ether (recovered). After the residue is cooled to room temperature, add 25ml of isopropanol and stir. Stir in an ice-water bath at 0°C. . After filtering, the filter cake was rinsed with a small amount of isopropanol and dried in vacuum to obtain 27.2 g (0.10 mol) of benzalide (general fo...
Embodiment 2
[0060] Example 2: Preparation of 2,3-dichlorobenzylidene acetoacetate methyl ester.
[0061] Add 108ml methyl tert-butyl ether into a 500ml reaction flask, and add 25g (0.14mol) 2,3-dichlorobenzaldehyde (general formula II), 0.65g piperidine, 0.5g glacial acetic acid, 20g under stirring at room temperature. (0.17mol) Methyl acetoacetate (Formula III). Increase the temperature and heat to 60-62°C to reflux, and start water separation. After 10 hours of timing reaction from the reflux, the heating was stopped and the temperature was cooled to room temperature. Wash with water to pH 6, dry the filtrate under reduced pressure to distill methyl tert-butyl ether (recovered) after drying with anhydrous calcium chloride, add 32ml of isopropanol to the residue after cooling to room temperature and stir well in an ice-water bath at 5°C Precipitation. After filtering, the filter cake was rinsed with a small amount of isopropanol and dried in vacuum to obtain 33.2 g (0.12 mol) of benzalid...
Embodiment 3
[0062] Example 3: Preparation of 2,3-dichlorobenzylidene acetoacetate methyl ester.
[0063] Add 117ml methylcyclohexane to a 500ml reaction flask, add 20g (0.11mol) 2,3-dichlorobenzaldehyde (general formula II), 0.65g piperidine, 0.5g glacial acetic acid, 25g ( 0.22mol) Methyl acetoacetate (general formula III). Raise the temperature and heat to 105~107℃ to reflux and start to separate water. The reaction time was 15 hours from the reflux, the heating was stopped, and the temperature was cooled to room temperature. Wash with water to pH 6, dry the filtrate under reduced pressure to remove methylcyclohexane (recovered). After the residue is cooled to room temperature, add 25ml of isopropanol and stir. Stir in an ice-water bath at 2°C for analysis. After filtering, the filter cake was rinsed with a small amount of isopropanol and dried under vacuum to obtain 25.4 g (0.09 mol) of benzalide (general formula IV) as a pale yellow solid substance with a yield of 84.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com