New preparation method of key intermediate of clevidipine butyrate
A technology of dichlorophenyl and pyridine carboxylic acid, applied in directions such as organic chemistry, can solve the problems of long reaction steps, high price, complicated operation, etc., and achieve the effects of mild reaction conditions, short reaction steps and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
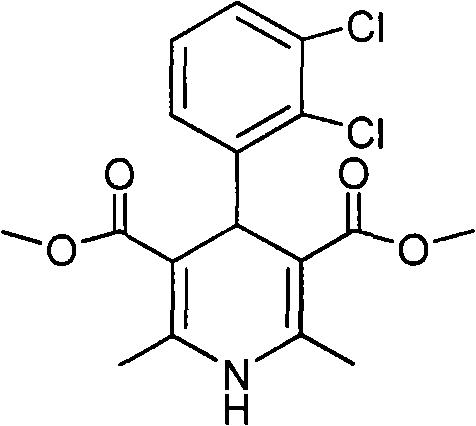
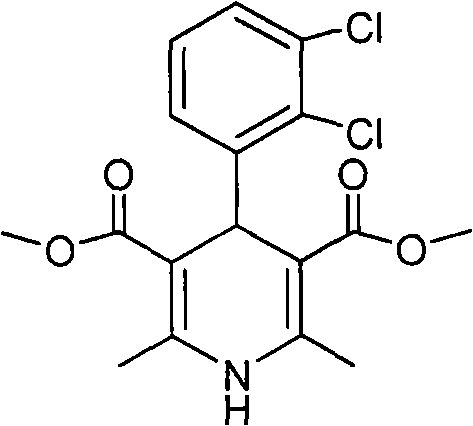
[0019] Methyl 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate (II)
[0020] 2,3-Dichlorobenzaldehyde (10.0g, 57mmol), methyl acetoacetate (13.3g, 114mmol) and 25% ammonia water (15.5g, 228mmol) were added to methanol (15ml), stirred, refluxed for 10h, and the reaction solution Cool to room temperature, filter with suction, and recrystallize the filter cake with 95% ethanol to obtain pale yellow crystal II (17.5 g, 82.7%) (document: 30.4%), mp 186-188°C. 1 HNMR (CDCl 3 ): 7.29-7.06 (m, 3H, Ar-H), 5.68 (brs, 1H, -NH), 5.47 (s, 1H, C 4 -H), 3.60(s, 6H, 2×-COOCH 3 ), 2.31(s, 6H, 2×-CH 3 ).
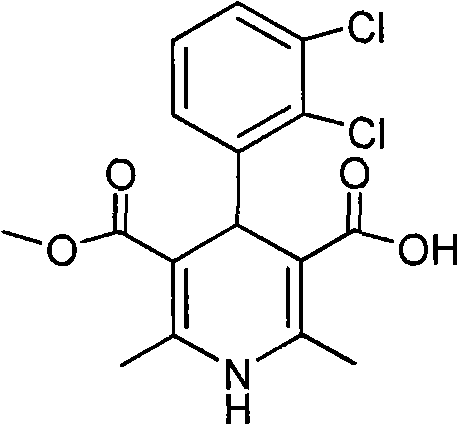
[0021] 4-(2,3-Dichlorophenyl)-1,4-dihydro-2,6-dimethyl-5-methoxycarbonyl-3-pyridinecarboxylic acid (I)
[0022] II (12.0g, 32mmol) was dissolved in methanol (173ml), added 40% NaOH solution (35.2ml), stirred, refluxed for 15h, the reaction solution was concentrated under reduced pressure to remove methanol, cooled to room temperature, added water (350ml), pumped ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com