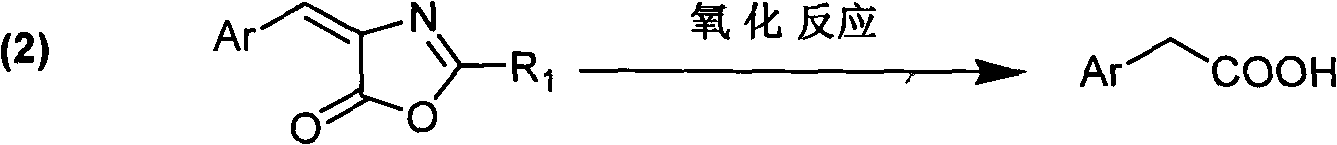
Preparation method for aryl acetic acid derivative
A technology of derivatives and aromatic groups, which is applied in the field of preparation of aromatic acetic acid derivatives, can solve problems such as shortage of raw material sources, troublesome post-processing, and large environmental pollution, and achieves suitable for large-scale industrial production, mild reaction conditions, The effect of little environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
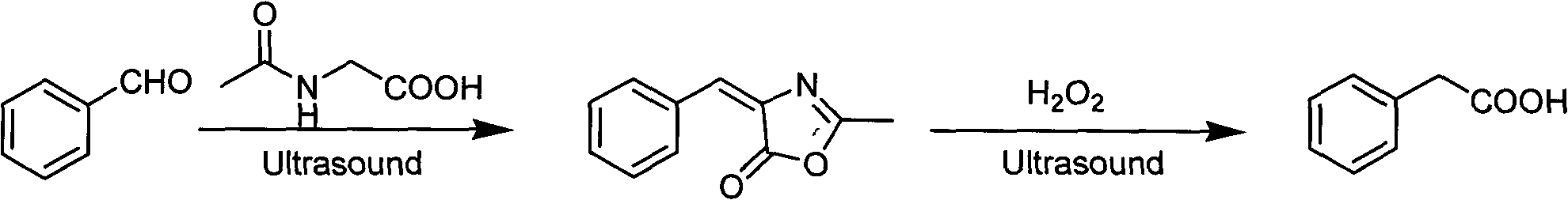
[0019] Embodiment 1: the preparation of phenylacetic acid
[0020] Weigh 10.6kg of benzaldehyde, 11.7kg of N-acetylglycine, 8.0kg of sodium acetate and 6.0kg of acetic anhydride in an ultrasonic reactor, react for 1 hour at a temperature of 80°C and a working frequency of 40kHz, and filter after cooling , add 7.1kg of 20% sodium hydroxide aqueous solution, mix evenly, pour 17.0kg of 30% hydrogen peroxide, react for 1h at a temperature of 20°C and a working frequency of 40kHz, filter to obtain crude phenylacetic acid, use petroleum After ether recrystallization, 8.9 kg of flaky phenylacetic acid crystals were obtained, with a content of 99.5%, a yield of 65%, and a melting point of 75-77°C. The nuclear magnetic resonance data of gained product is: 1 H NMR (500MHz, CDCl 3 ): δ3.52 (s, 2H, -CH2-), 7.14 to 7.19 (m, 5H, H-Ph), 11.17 (s, IH, COOH).
[0021] The reaction formula is as follows:
[0022]
Embodiment 2
[0023] Embodiment 2: the preparation of phenylacetic acid
[0024] Weigh 3.2kg of benzaldehyde, 3.5kg of N-acetylglycine, 2.4kg of sodium acetate and 1.8kg of acetic anhydride in a round-bottomed flask, reflux at 100°C for 2 hours, filter after cooling, and add 20% hydroxide 3.2 kg of sodium aqueous solution, after mixing evenly, pour 5.1 kg of 30% hydrogen peroxide, stir and react at 40° C. for 2.5 h, filter to obtain crude phenylacetic acid, recrystallize with petroleum ether to obtain 0.8 kg of flaky phenylacetic acid crystals, The content is 99.3%, the yield is 48%, and the melting point is 75-77°C. The nuclear magnetic resonance data of gained product is: 1 H NMR (500MHz, CDCl 3 ): δ3.52 (s, 2H, -CH2-), 7.14 to 7.19 (m, 5H, H-Ph), 11.17 (s, IH, COOH).
[0025] The reaction formula is as follows:
[0026]
Embodiment 3
[0027] Embodiment 3: Preparation of p-nitrophenylacetic acid crystals
[0028] Weigh 13.6kg of p-nitrobenzaldehyde, 10.5kg of N-acetylglycine, 7.0kg of sodium acetate and 5.4kg of acetic anhydride and place them in an ultrasonic reactor, and react for 1 hour at a temperature of 70°C and a working frequency of 55kHz. After cooling, filter, add 7.1kg of 5% sodium hydroxide aqueous solution, mix evenly, pour 15.3kg of 30% hydrogen peroxide, react for 1h at a temperature of 25°C and a working frequency of 55kHz, and filter to obtain p-nitro The crude product of phenylacetic acid was recrystallized with hot water to obtain 10.2 kg of p-nitrophenylacetic acid crystals, with a content of 99.3%, a yield of 68%, and a melting point of 153-154°C. The nuclear magnetic resonance data of gained product is: 1 H NMR (500MHz, CDCl 3 ): δ3.38(s, 2H, -CH2-), 7.34~7.35(d, J=8.2Hz, 2H, H-Ph), 7.43~7.45(d, J=7.5Hz, 2H, H-Ph) , 11.11 (s, IH, COOH).
[0029] The reaction formula is as follows: ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com