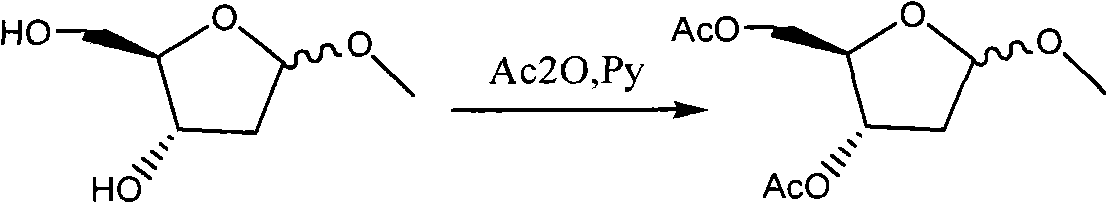Preparation method of decitabine
A decitabine and compound technology, applied in the field of drug preparation, can solve the problems of low total yield, difficult industrialization and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 1-methyl--2-deoxy-D-ribose (formula II)
[0031] 2-Deoxy-D-ribose (136 g, 1 mol) was added to methanol (500 ml), concentrated sulfuric acid (2 ml) was added dropwise, and stirred at room temperature for 2 hours. Sodium carbonate (4 g) was then added to neutralize. After suction filtration, the filtrate was evaporated to dryness under reduced pressure to obtain the compound of formula II, which was directly used in the next reaction.
Embodiment 2
[0032] Example 2 1-methyl-3,5-diacetoxy-2-deoxy-D-ribose (Formula III)
[0033] Formula II was dissolved in pyridine (500ml, 6mol), cooled to 0°C, slowly added dropwise with acetic anhydride (210ml, 3mol), reacted at room temperature for 12h, added dropwise with water (350ml), dichloromethane (1000ml), and stirred for 2h. Separation, the organic phase was successively washed with water (350ml), 2N HCl (350ml), saturated sodium bicarbonate (350ml), dried over anhydrous sodium sulfate, filtered and evaporated to dryness to obtain oily formula III (231.2g), yield 90% ( Calculated as 2-deoxy-D-ribose).
[0034] 1 H-NMR (CDCl 3 )6.0(t, 1H), 5.2(s, 1H), 5.1(t, 1H), 5.07(d, 1H), 5.0(t, 1H), 4.3-4.1(m, 6H), 3.4(s, 1H ), 3.3(s, 1H), 2.3(m, 2H), 2.1(m, 2H)
[0035] Gas Chromatography Conditions
[0036]
Embodiment 33
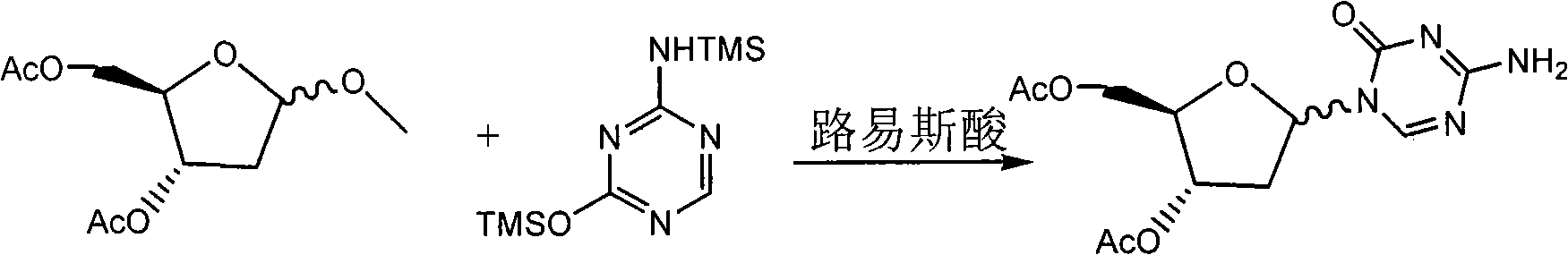
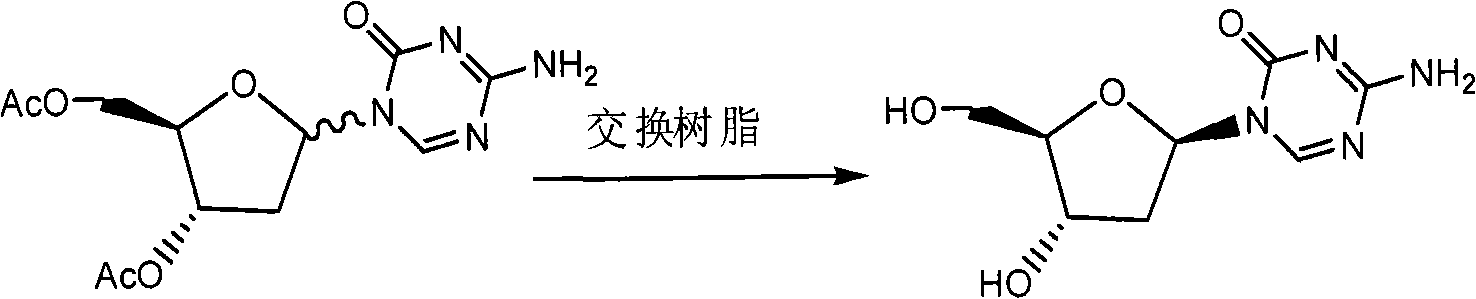
[0037] Example 33, 5-diacetoxy-5-aza-2'-deoxycytidine (Formula V)
[0038] Add 5-azacytosine (50.7g, 452.1mmol), hexamethyldisilazane (500ml, 2.4mol), ammonium sulfate (2g) in the 1000ml three-necked flask, heat and reflux under nitrogen to clarification, evaporate under reduced pressure The solvent was removed to obtain 2,4-bis-(trimethylsilyl)-5-azacytosine (Formula IV). Formula IV was dissolved in 1,2-dichloroethane (250ml).
[0039] Formula III (100g, 430.6mmol) was dissolved in 1,2-dichloroethane (500ml), then dropped into the above-mentioned 1,2-dichloroethane solution of formula IV, and anhydrous tetrachloride was added dropwise at 5°C Tin (52.5ml, 452.1mmol), stirred overnight at room temperature. Wash with saturated sodium bicarbonate solution dropwise until neutral, suction filter, wash the filtrate with saturated sodium bicarbonate solution (500ml), separate liquids, dry the organic phase with anhydrous sodium sulfate, filter and evaporate to dryness to obtain form...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com