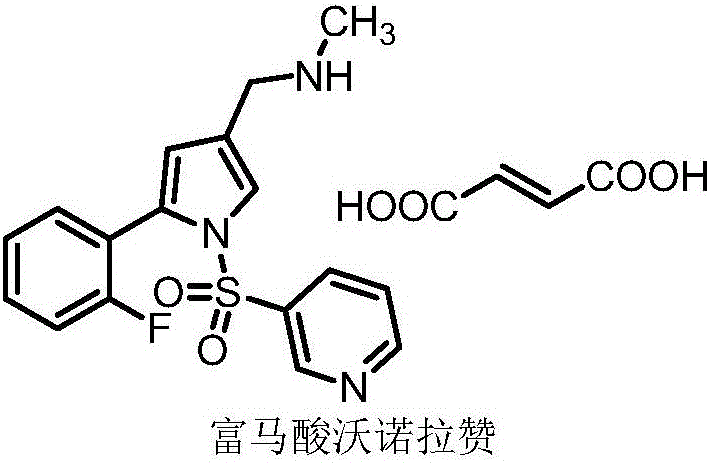
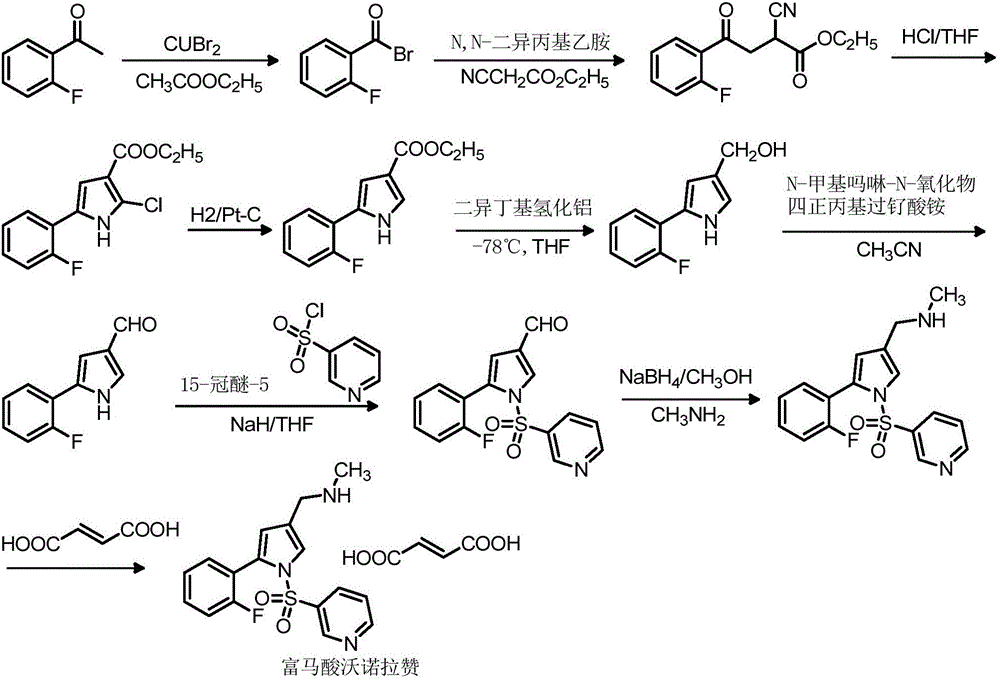
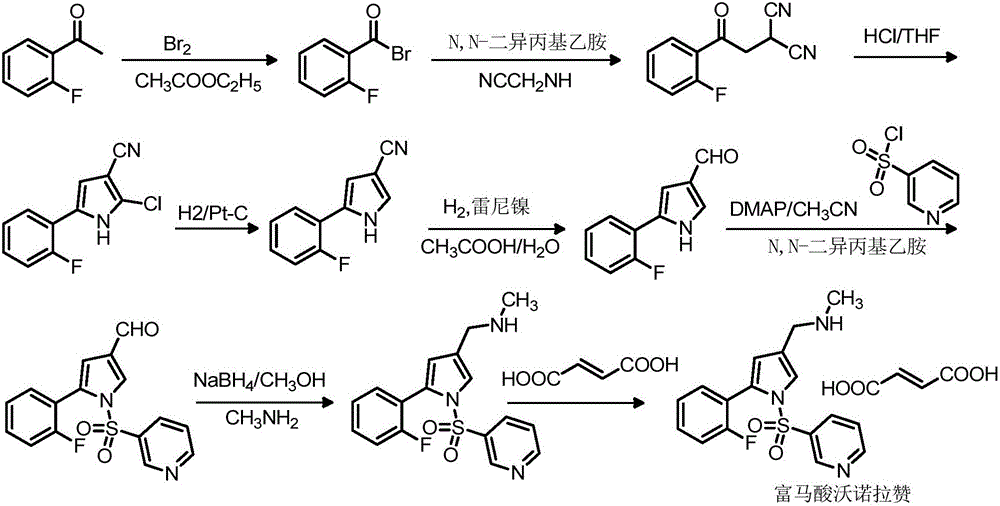
Vonoprazan fumarate midbody, preparation method thereof and method for preparing vonoprazan fumarate midbody
A technology for vornorazan fumarate and an intermediate, which is applied in the field of synthesis of vornorazan fumarate, can solve the problems of low yield, cumbersome operation, unfavorable safe production and the like, and achieves high yield and mild conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
[0075] Reference example 1N-Methyl-2-cyano-4-(2-fluorophenyl)-4-oxobutyramide (fumaric acid wonorazan intermediate I)
[0076] Its structure is as follows:
[0077]
[0078] Vonoprazan fumaric acid intermediate I is prepared by the following method:
[0079] Add 95g of o-fluoroacetophenone and 500mL of ethyl acetate into a dry 2000mL three-neck flask, slowly add the mixture of 120g of bromine and 300mL of ethyl acetate dropwise at 25°C under stirring, control the dropwise addition within 1 hour, and the temperature does not exceed 35°C After the dropwise addition, keep warm at 25°C for 1.5h, then add 3% anhydrous sodium sulfite solution dropwise; after the addition, keep warm at 25°C for 1.5h; adjust the pH to 7 with sodium bicarbonate and then separate the layers, and wash the organic phase with saturated brine for 2 times, each 100mL; get 2-bromo-1-(2-fluorophenyl) ethyl ketone acetate solution;
[0080] Cool the 2-bromo-1-(2-fluorophenyl) ethyl ketone acetate solution ...
Embodiment 1
[0081] Example 1N-methyl-5-(2-fluorophenyl)-1H-pyrrole-3-carboxamide (fumaric acid vornorazan intermediate II)
[0082] Its structure is as follows:
[0083]
[0084] The preparation method of the fumaric acid vornorazan intermediate II is as follows:
[0085] In a dry 10L three-necked flask, add 10g of vonorazan fumaric acid intermediate I and 300mL of tetrahydrofuran successively, add 1g of 5% palladium carbon under stirring, then add 300mL of glacial acetic acid and 2g of anhydrous sodium sulfate; Replace continuously for 3 times, then replace with hydrogen continuously for 3 times, start timing and add hydrogen, the reaction temperature is 20-35°C; after the detection reaction is completed, replace hydrogen with nitrogen for 4 times, then filter, the filtrate cools below 10°C, drop Add 150mL of water, filter, collect the filter cake, and dry to obtain 7.2g of fumaric acid vonoprazan intermediate II, yield: 77.3%; 1 H-NMR (400MH Z , DMSO-d 6 )δ (ppm): 2.81 (s, 3H), 6...
Embodiment 2
[0086] Example 2N-methyl-1-[5-(2-fluorophenyl)-1H-pyrrol-3-yl]-N-methylamine (Vonoprazan fumaric acid intermediate III)
[0087] Its structure is as follows:
[0088]
[0089] The preparation method of this fumaric acid vornorazan intermediate III is as follows:
[0090] Dissolve 43g of iodine in 70mL of tetrahydrofuran to make a solution; add 110mL of tetrahydrofuran and 22.5g of vonoprazan fumaric acid intermediate II into the reaction flask, replace with nitrogen, stir, and cool down; add 14g of sodium borohydride in batches, control The temperature is -5-15°C, add iodine tetrahydrofuran solution dropwise, control the system temperature at -5-15°C, after the dropwise addition, raise the temperature to 15-25°C, stir for 1-1.5h; raise the temperature to 55-65°C for heat preservation reaction , react for 3 hours, cool down to -5-5°C, add dropwise 50mL of 4N hydrochloric acid to quench the reaction, control the temperature at -5-20°C; raise the temperature of the system to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com