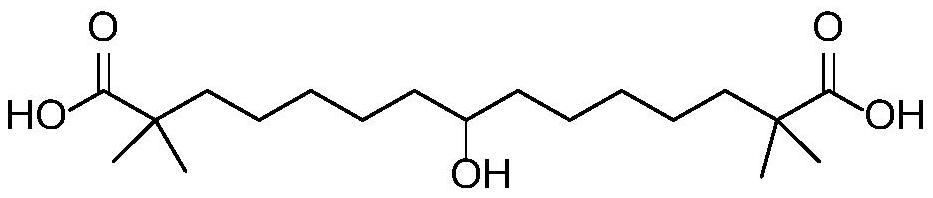
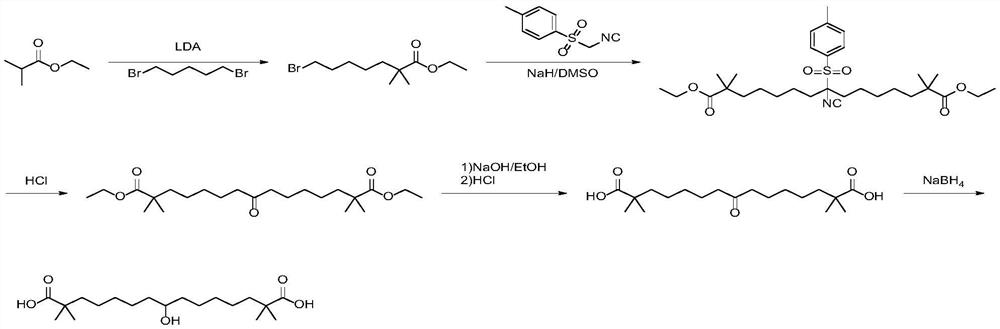
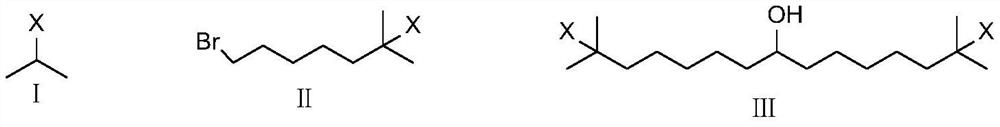Synthetic method of bempedoic acid
A synthesis method and compound technology, applied in chemical instruments and methods, carboxylic acid nitrile preparation, carboxylic acid ester preparation, etc., can solve problems such as difficult to obtain reaction raw materials, difficult to carry out designed reactions, difficult to carry out coupling reactions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The first step: the preparation of 7-bromo-2,2-dimethylheptanitril
[0050]Under the protection of nitrogen, 50g (0.72mol, 1.0eq) of isobutyronitrile and 300mL of tetrahydrofuran were added to a 1000mL four-necked flask, and the temperature of the reaction system was slowly lowered to -20°C, and 86.8g (2.17mol, 3.0eq), control the temperature of the reaction system not to exceed -10°C, continue to stir the reaction at -20°C for 1 hour after the dropwise addition, slowly add 166g (0.72mol, 1.0eq) of 1,5-dibromopentane dropwise, During the dropwise addition, the reaction temperature was controlled not to exceed -10°C. After the dropwise addition, the temperature of the reaction was slowly raised to room temperature, and the reaction was stirred overnight, and the conversion of the raw materials was detected by GC. The reaction solution was quenched with saturated ammonium chloride, extracted with ethyl acetate (500 mL×3), the organic phases were combined, dried over anhyd...
Embodiment 2
[0059] The first step of preparation of 7-bromo-2,2-dimethylheptanoic acid methyl ester
[0060] Under nitrogen protection, add 74g (0.72mol, 1.0eq) of methyl isobutyrate and 300mL of tetrahydrofuran into a 1000mL four-neck flask, slowly lower the temperature of the reaction system to -20°C, and add 86.8g of sodium hydride (2.17 mol, 3.0eq), control the temperature of the reaction system not to exceed -10°C, continue to stir the reaction at -20°C for 1 hour after the dropwise addition, and slowly add 166g of 1,5-dibromopentane (0.72mol, 1.0eq ), the reaction temperature was controlled not to exceed -10°C during the dropwise addition. After the dropwise addition, the temperature of the reaction was slowly raised to room temperature, and the reaction was stirred overnight (12 hours), and the conversion of the raw materials was detected by GC. The reaction solution was extracted with saturated ammonium chloride, extracted with 500mL*3 ethyl acetate, the organic phases were combin...
Embodiment 3~5
[0069] Same condition as embodiment 1, just replace the alkali in the first step with following components, experimental result is as shown in table 1:
[0070] Table 1
[0071] Example alkali Yield of the first step reaction (%) Purity of the first step reaction (%) Example 3 Potassium tert-butoxide 85.1 98.4 Example 4 lithium diisopropylamide 94.6 99.1 Example 5 Lithium bis(trimethylsilyl)amide 90.8 98.5
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com