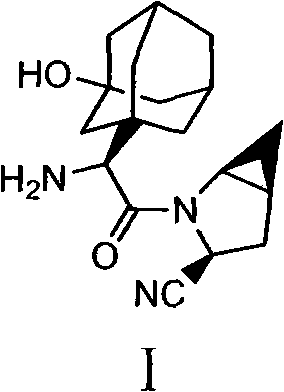
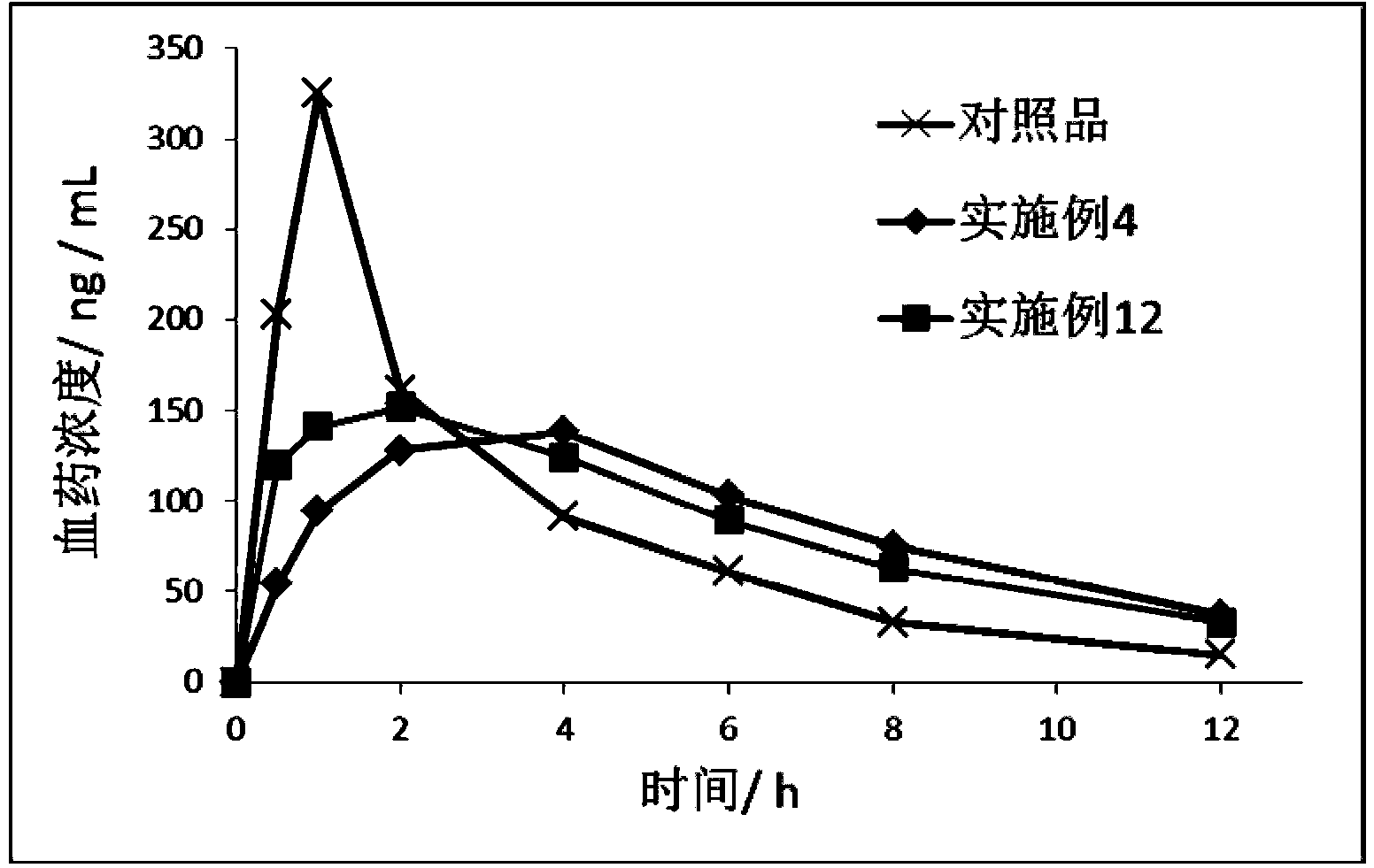
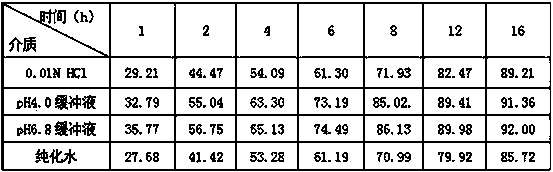
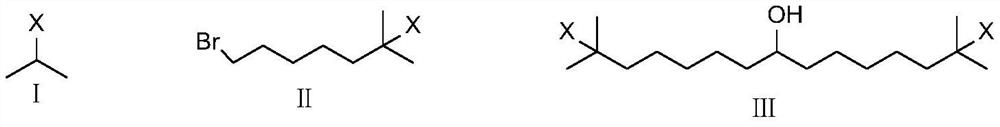Patents
Literature
64results about How to "Suitable for process production" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dasatinib polymorphic substance as well as preparation method and medicinal composition thereof
ActiveCN102086195ASimple processHigh purityOrganic active ingredientsNervous disorderDasatinibIndustrial scale
The invention discloses a dasatinib polymorphic substance. In addition, the invention also discloses a preparation method and a medicinal composition of the dasatinib polymorphic substance. The dasatinib polymorphic substance provided by the invention has the advantages of good physicochemical property and good stability, is more suitable for industrial scale preparation, and the like.
Owner:NANJING CAVENDISH BIO ENG TECH +1
Method for synthesizing trifluoroiodomethane and pentafluoroethyliodide meanwhile
ActiveCN101219925AConvenient sourceImprove stabilityHalogenated hydrocarbon preparationChemical synthesisAlkaline earth metal
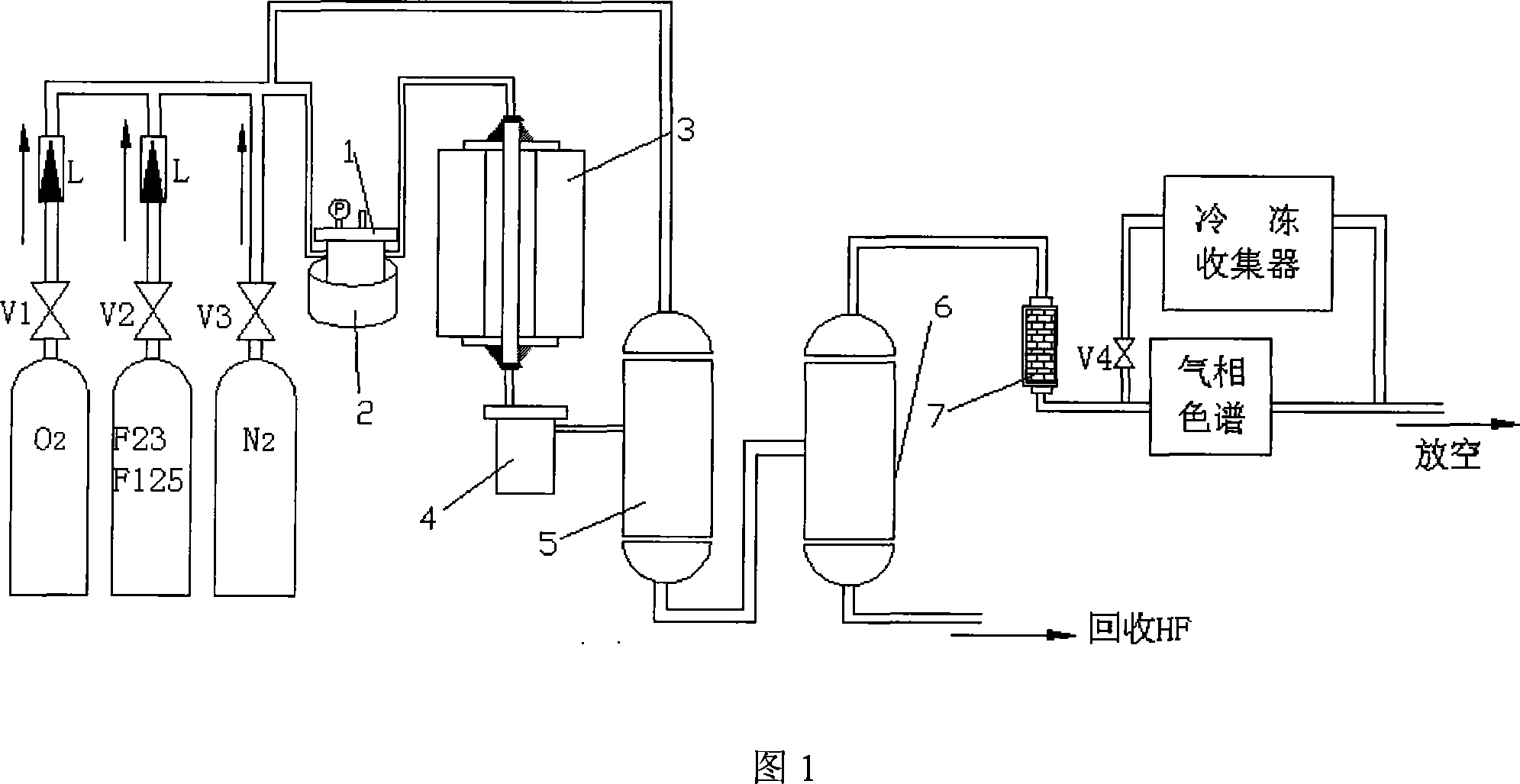
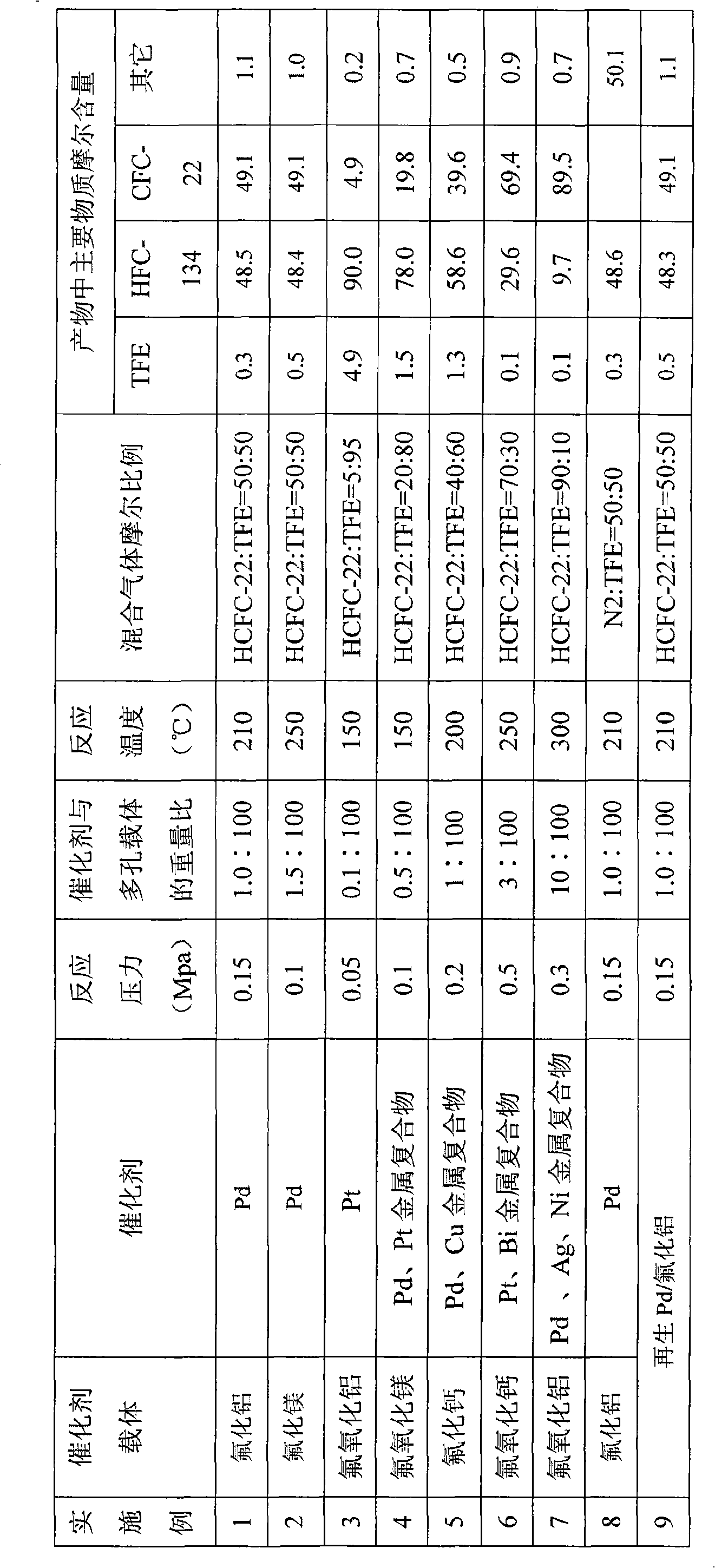
The invention relates to a method of synthesizing CF_3I and C2F5I simultaneously, which belongs to the filed of organic chemical synthesis. The method of synthesizing CF_3I and C2F5I simultaneously is characterized in that a alkali metal salt, a alkali earth metal salts or / and a copper salt are taken as a catalyst to be attached to a porous carrier; an oxygen or mixed gas of oxygen and an inert gas is pumped; a CHF3 and / or a HFC_125 are taken as a raw material; an iodine is taken as an iodinating agent; then iodination occurs in a reactor under high temperature to generate the C2F5I and the CF_3I. The porous carrier is a fluoride or an oxyfluoride. The raw material of the invention is nontoxic and is convenient to get; the catalyst does not react with a reaction medium and has good stability. The synthesis process is safe and suitable for technical production.
Owner:泉州宇极新材料科技有限公司
Trimetazidine dihydrochloride sustained-release tablet and preparation method thereof
InactiveCN102885795AThe solution is not easy to cleanSimple preparation stepsOrganic active ingredientsPharmaceutical non-active ingredientsTrimetazidine DihydrochlorideSustained Release Tablet
The invention discloses a trimetazidine dihydrochloride sustained-release tablet and a preparation method thereof. The trimetazidine dihydrochloride sustained-release tablet comprises the following constituents in percentage by mass: 5-60% of trimetazidine dihydrochloride, 10-25% of sustained-release framework material, 1-8% of adhesive, 20-80% of filler, 0.1-5% of glidant and 0.2-3% of lubricant. According to the trimetazidine dihydrochloride sustained-release tablet, medicine can be slowly and uniformly released by adding the sustained-release framework material, so as to achieve regulation and control for a release speed, reduce the peak-valley ratio of the medicine, improve the efficacy, reduce the toxic and side effects of the medicine, reduce daily medicine-taking times and enhance the compliance of the patient on the medicine. The preparation method of the trimetazidine dihydrochloride sustained-release tablet disclosed by the invention is simple in process, does not need specially process production equipment, and is low in cost and good for batch amplification and industrialized production for products.
Owner:AC PHARMA CO LTD
Medicinal salts of saxagliptin and preparation methods of medicinal salts
InactiveCN102086172AGood water solubilityImprove oral bioavailabilityOrganic active ingredientsOrganic chemistrySaxagliptinDiabetes mellitus
The invention provides medicinal salts of saxagliptin, and particularly relates to mesylate, meleate, malate, succinate and citrate as well as preparation methods of the medicinal salts. The medicinal salts have the advantage of good stability, and are not easy to degrade. Preparations with controllable quality and low cost, which are suitable for industrial production, are prepared from the medicinal salts and pharmaceutically acceptable carriers through a conventional preparation technology. The medicinal salts can be used for treating and / or preventing diabetes mellitus.
Owner:廖国超
Immunomodulator slow-release preparation and preparation method thereof
ActiveCN103610658AProlong the action timeUniform and constant action timeOrganic active ingredientsPill deliveryBlood concentrationProlonged-release tablet
The invention discloses an immunomodulator slow-release preparation and a preparation method thereof. A lenalidomide slow-release tablet is composed of a slow-release layer and an optional quick-release layer, wherein the slow-release layer contains active ingredients of lenalidomide and a slow-release framework material simultaneously; the quick-release layer does not contain the slow-release framework material. The lenalidomide slow-release tablet disclosed by the invention is capable of slowly and uniformly releasing medicines by virtue of the slow-release framework material, so as to reduce the release speed, delay the time to peak, prolong the action time of lenalidomide, and provide a uniform and constant blood concentration. Moreover, The lenalidomide slow-release tablet disclosed by the invention is simple in prescription and excellent in quality stability; the preparation process is simple to operate, free from special treatment and production equipment, low in production cost, and beneficial to batch-enlarged industrial production for the product; the preparation method is high in yield, the granulation and crushing procedures are simple and practicable to operate, the intermediate material is good in stability, flowability, compressibility and content uniformity, and completely meets the requirements of tabletting, and the surface of the prepared tablet is smooth and beautiful.
Owner:AC PHARMA CO LTD
Imatinib mesylate polymorph and pharmaceutical composition
ActiveCN102070605ANot suitable for the needs of large-scale and stable productionSimple preparation processOrganic active ingredientsSenses disorderImatinib mesylateMedicine
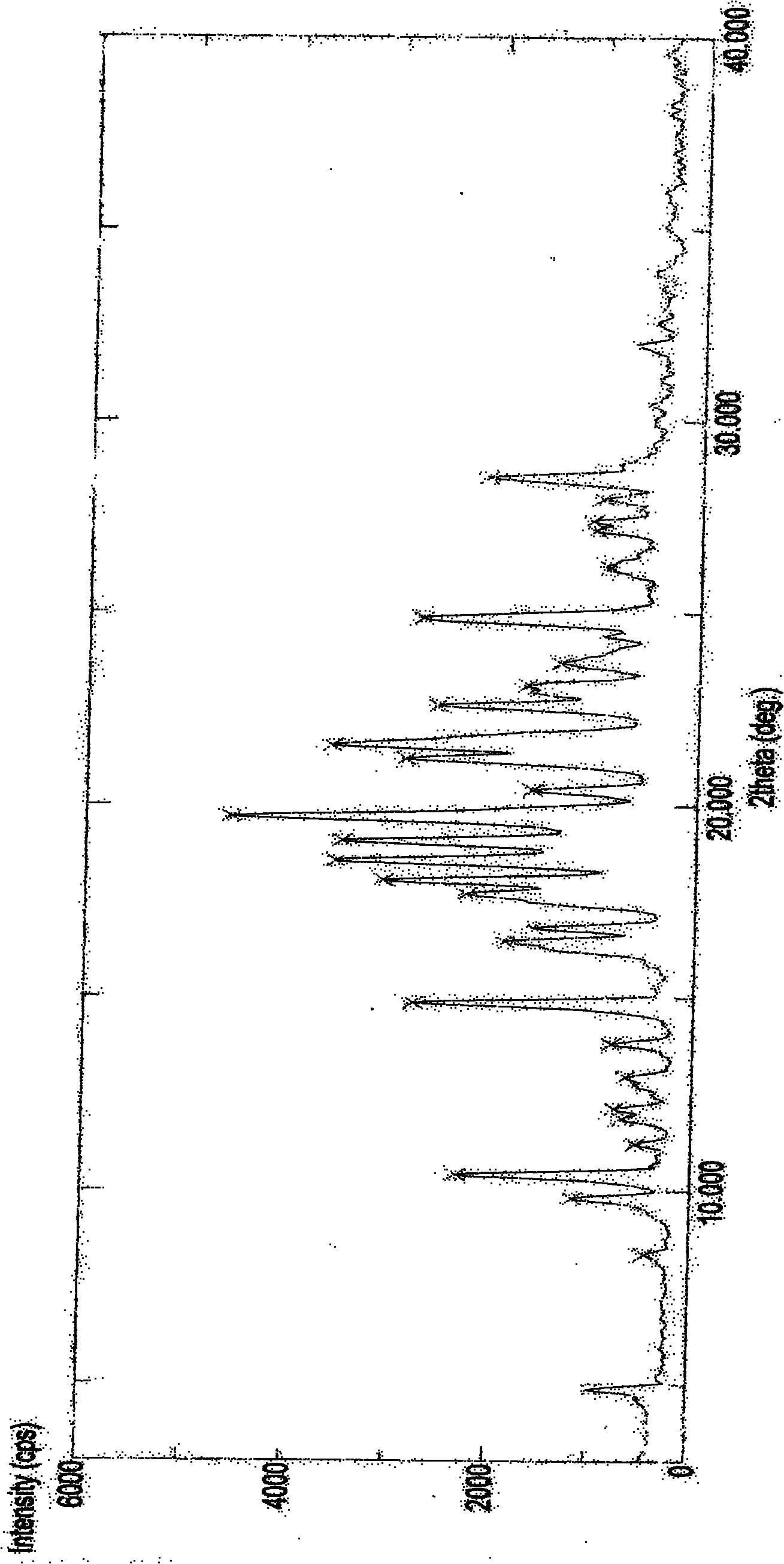
The invention discloses an imatinib mesylate polymorph I, which is a typical X-ray diffraction pattern and has a diffraction peak at 2theta of 17.7+ / -0.2 degrees, 18.1+ / -0.2 degrees, 18.6+ / -0.2 degrees, 19.1+ / -0.2 degrees, 19.7+ / -0.2 degrees and 20.4+ / -0.2 degrees. In addition, the invention also discloses a preparation method thereof and a pharmaceutical composition. The imatinib mesylate polymorph I has the advantages of high purity, excellent physicochemical property and high stability, and is more suitable for industrial mass production.
Owner:NANJING CAVENDISH BIO ENG TECH +1
High-rate lithium ion battery graphite cathode material and preparation method thereof
InactiveCN109437184AImprove performanceIncrease charge and discharge rateCarbon compoundsHybrid capacitor electrodesHigh rateSurface oxidation
The invention belongs to the field of lithium ion batteries, and particularly relates to a high-rate lithium ion battery graphite cathode material and a preparation method thereof. The preparation method comprises the following steps: (1) pulverization and shaping of raw materials; (2) graphitization; (3) demagnetization screening; (4) liquid phase coating granulation; (5) surface oxidation treatment. According to the high-rate lithium ion battery graphite cathode material and the preparation method thereof, a liquid phase coating granulation process replaces traditional secondary granulationthrough optimized fine graphite, so as to realize uniform coating of the surface of graphite, reduce the anisotropism of the graphite and shorten the diffusion path of lithium ions; then a micro porestructure is formed on the surface of the graphite through a surface oxidization pore formation process, so that a lithium ion migration channel of a cardinal plane is added to increase the migrationspeed of lithium ions. The charging rate of the graphite obtained by the method is more than 8 C, and the capacity can be maintained at 80 percent or above under the discharging rate more than 30 C.
Owner:KEDA (ANHUI) NEW MATERIAL CO LTD
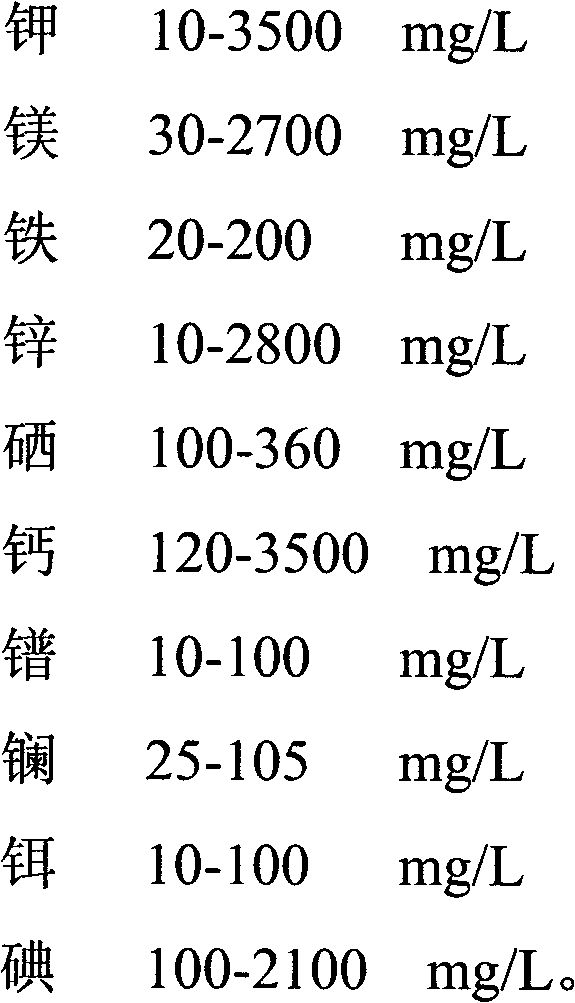
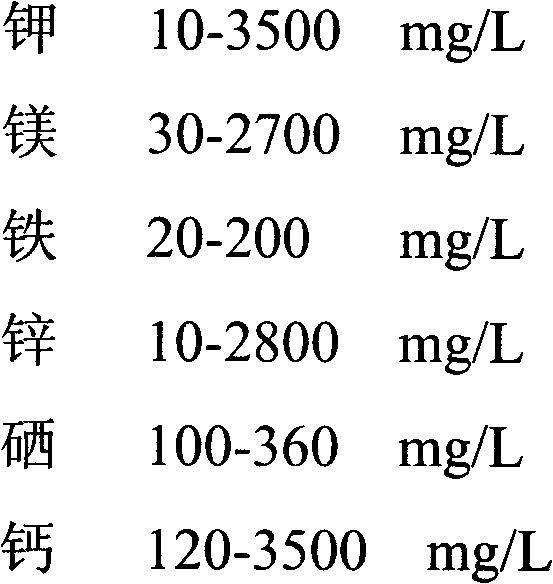
Method for improving yield and trace element enrichment of liquid fermentation mycelium of lucid ganoderma
The invention relates to the field of liquid fermentation mycelium of lucid ganoderma and provides a method for improving yield and trace element enrichment of the liquid fermentation mycelium of the lucid ganoderma. The method is characterized by comprising the steps of inoculating a slope lucid ganoderma strain onto a liquid seed culture medium added with trace element-containing substances, performing mutation breeding by ultraviolet rays and cobalt-60 gamma rays, placing the lucid ganoderma mycelium into a fermentation tank for expansion breeding step by step, and performing separation filtering, vacuum microwave drying and crushing on the lucid ganoderma mycelium to obtain a product. The method adopts the fermentation process controlled by adopting pH value steady state feedback step by step according to the composition and distribution of the trace elements in the culture medium, thus more facilitating improving the conversion efficiency of multiple trace elements; the process is efficient and controllable, and the mycelium is high in additional value and suitable for process production; and the drying method for the mycelium adopts hot wind combined with a vacuum microwave drying technology and keeps the biological activity of effective constituents in the mycelium.
Owner:HUBEI JIAFU BIOLOGICAL TECH
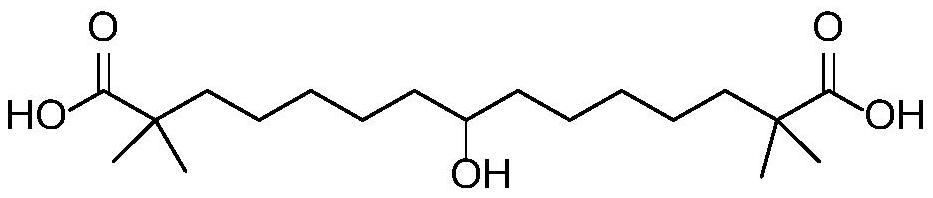
Synthetic method of bempedoic acid
PendingCN111825546AShort reaction stepsHigh yieldOxygen-containing compound preparationCarboxylic acid nitrile preparationBempedoic acidGrignard reagent
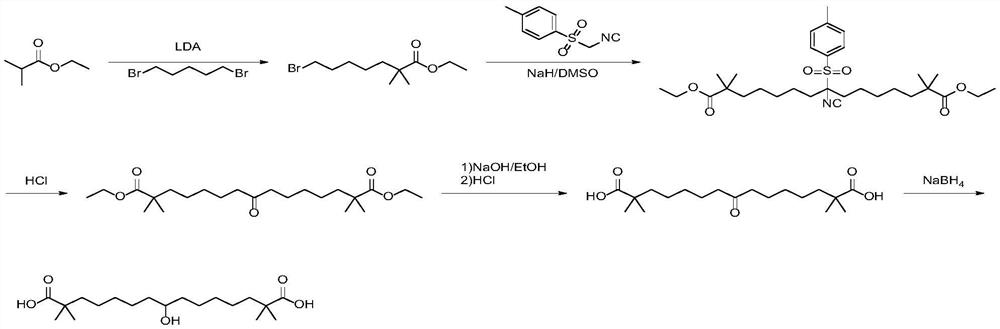
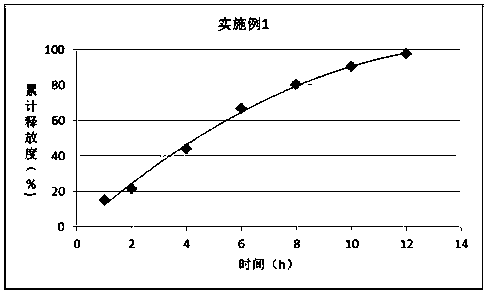
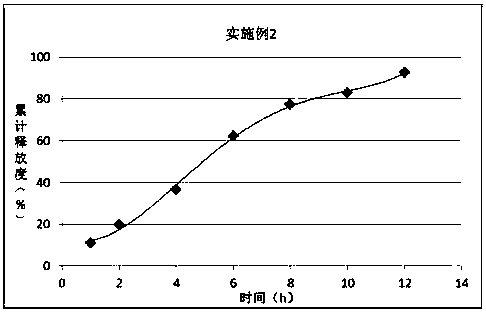
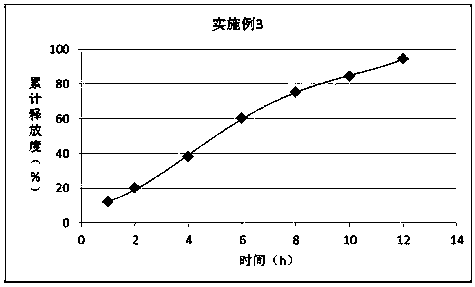
The invention discloses a synthetic method of bempedoic acid. The method comprises the following steps of: taking isobutyronitrile (ester) as a starting raw material, carrying out reaction with 2, 5-dibromopentane under the catalysis of alkali to generate 7-bromo-2, 2-dimethylheptonitrile (ester), then forming a Grignard reagent with magnesium, carrying out reaction with formate to generate 8-hydroxy-2, 2, 14, 14-tetramethylpentadecane dinitrile (ester), and finally carrying out alkaline hydrolysis and acidification to obtain bempedoic acid. The synthetic route is short, all the used raw materials are easy to obtain, the cost is low, the yield of each step of reaction is high, and the purity is high, therefore the method is suitable for industrial production.
Owner:合肥市梓熤科技贸易有限公司
Pharmaceutical composition of ornithine aspartate
InactiveCN108524453AImprove complianceStable blood concentrationOrganic active ingredientsPowder deliveryDrugPatient compliance
The invention discloses a pharmaceutical composition of ornithine aspartate. By fusing, mixing and preparing ornithine aspartate and a proper amount of PLGA and PEG6000, the ideal slow-release effectcan be achieved. The pharmaceutical preparation prepared from the pharmaceutical composition of ornithine aspartate is slowly released, a patient just needs to take the medicine once a day, the patient compliance is high, the stable blood concentration can be maintained, the side effect is small, and the effect is good; the consumption of auxiliary materials is low, the preparation technology is simple, and the composition is suitable for technological production.
Owner:YANGTZE RIVER PHARM GRP GUANGZHOU HAIRUI PHARM CO LTD +1
6-halogenated glucose C-glycoside as well as preparation method and application thereof
ActiveCN108675976AHigh purityHigh yieldOrganic chemistryBulk chemical productionC-glycosideD-Glucose
The invention discloses a 6-halogenated glucose C-glycoside as well as a preparation method and application thereof. A structure of 6-halogenated glucose C-glycoside is shown in formula I; an intermediate can be synthesized efficiently with cheap and easily available raw materials; meanwhile, when the raw material is used for synthesizing Jardiance, dapagliflozin and the like, a reaction yield ishigh, and an obtained product has high purity and relatively high industrial application prospect.
Owner:ZHEJIANG HONGYUAN PHARMA
Imatinib mesylate polymorph and pharmaceutical composition thereof
ActiveCN102260242AHigh purityGood physical and chemical propertiesOrganic active ingredientsNervous disorderMedicineImatinib mesylate
Owner:NANJING CAVENDISH BIO ENG TECH +1
Synthetic method of pitavastatin tert-butyl ester
ActiveCN111875538AHigh stereoselectivityHigh purityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystQuinoline
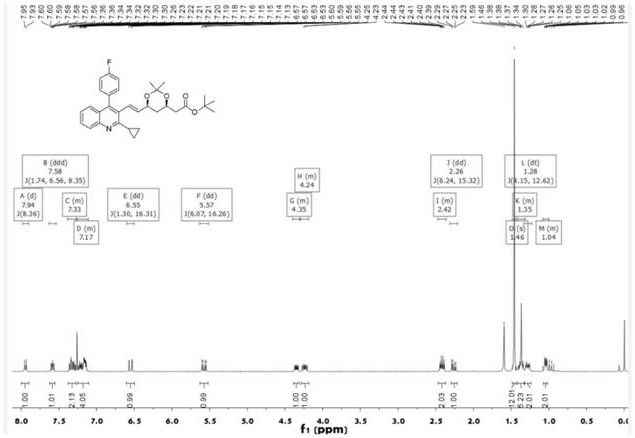
The invention discloses a synthesis method of pitavastatin tert-butyl ester, which comprises the following steps: carrying out a reaction on (4R-Cis)-6-chloromethyl-2,2-dimethyl-1,3-dioxolane-4-tert-butyl acetate with a substance A under the action of a first base catalyst to obtain a substance B; oxidizing the substance B with an oxidizing agent to obtain a substance C; carrying out a reaction with 2-Cyclopropyl-4-(4-fluorophenyl)-quinoline-3-formaldehyde under the action of a second base catalyst to obtain a substance D; and finally, performing acid deprotection to obtain the pitavastatin tert-butyl ester. The method has the advantages of mild and controllable reaction conditions, wherein the reaction conditions of Julia olefination are free of requirement of ultralow-temperature reaction, so that the synthesis method has advantages of convenience and simplicity in operation, good stereoselectivity and high yield, and the synthesized pitavastatin tert-butyl ester is completely free of cis-isomer and high in purity.
Owner:ANHUI QINGYUN PHARMA & CHEM
Method for synthesizing 1,1,2,2-tetrafluoroethane
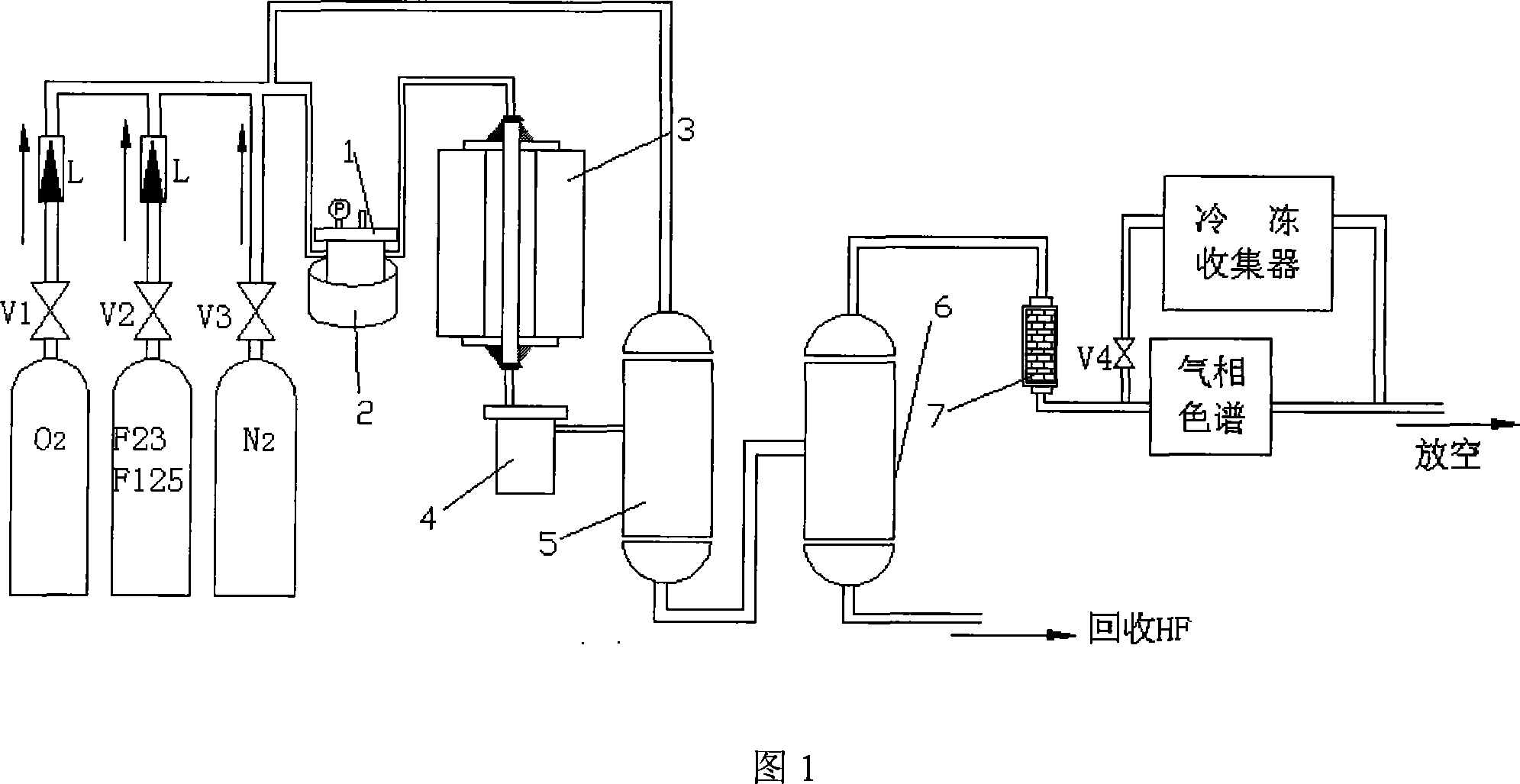
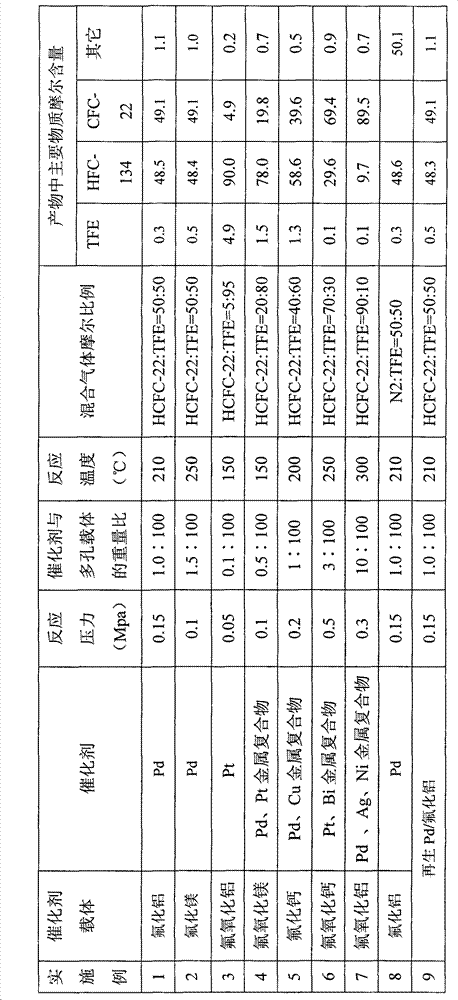
ActiveCN101591218AEasy to recycle and recycleExtended service lifeCatalyst carriersHalogenated hydrocarbon preparationHydrogen fluorideHydrogen
The invention relates to a method for synthesizing 1,1,2,2-tetrafluoroethane, which belongs to the field of preparation of refrigerants. TFE-containing mixed gas and hydrogen pass through a porous carrier supported hydrogenation catalyst bed to generate HFC-134; the TFE-containing mixed gas is a mixture formed by TFE and inert gas; the inert gas is gas which does not react with the TFE and the hydrogen under reaction conditions; and a porous carrier material is fluoride or oxyfluoride. An inert component in the method does not participate in the reaction, but can take away the heat generated by the reaction to ensure that the temperature of the catalyst bed is relatively steady and the service life of a catalyst is prolonged; and the adopted porous carrier is the fluoride or the oxyfluoride, does not react with hydrogen fluoride removed during the reaction, improves the service life of the catalyst and is easy to recycle at the same time. The synthesis method has good stability and safe synthesis process, and is suitable for technologized production.
Owner:泉州宇极新材料科技有限公司
Racecadotril double-phase releasing preparation and preparation method thereof
ActiveCN106822907AIncrease contactHigh dissolution rateOrganic active ingredientsDigestive systemRacecadotrilDouble phase
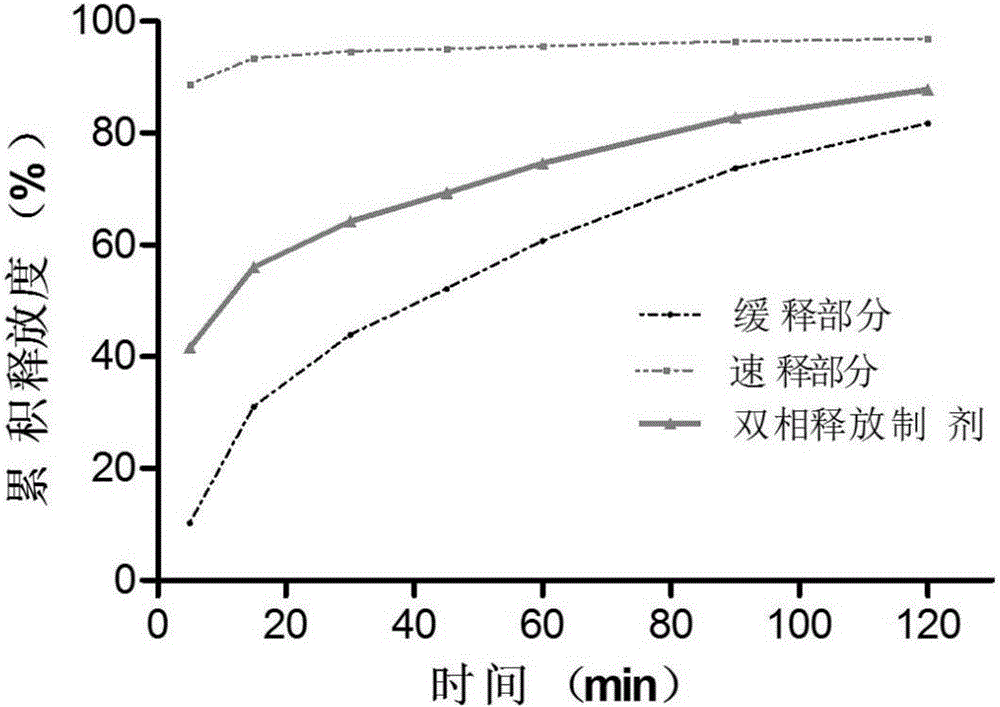
The invention relates to the field of medicines, in particular to a racecadotril double-phase releasing preparation and a preparation method thereof. The racecadotril double-phase releasing preparation is prepared from medicine-containing particles with different slow-release properties and the like in percentage by mass: 10 to 90% of slow-release medicine-containing particles, 10 to 90% of quick-release medicine-containing particles, 0.1 to 0.5% of lubricant, and 0.1 to 1.0% of flavoring agent. The racecadotril double-phase releasing preparation has the advantages that the bitter taste of the racecadotril is effectively covered, the effective plasma concentration is guaranteed, the preparation technology is simple, the reproducibility is good, and the racecadotril double-phase releasing preparation is suitable for industrialized production.
Owner:SHANDONG DYNE MARINE BIOTECHCAL PHARM HLDG CO LTD
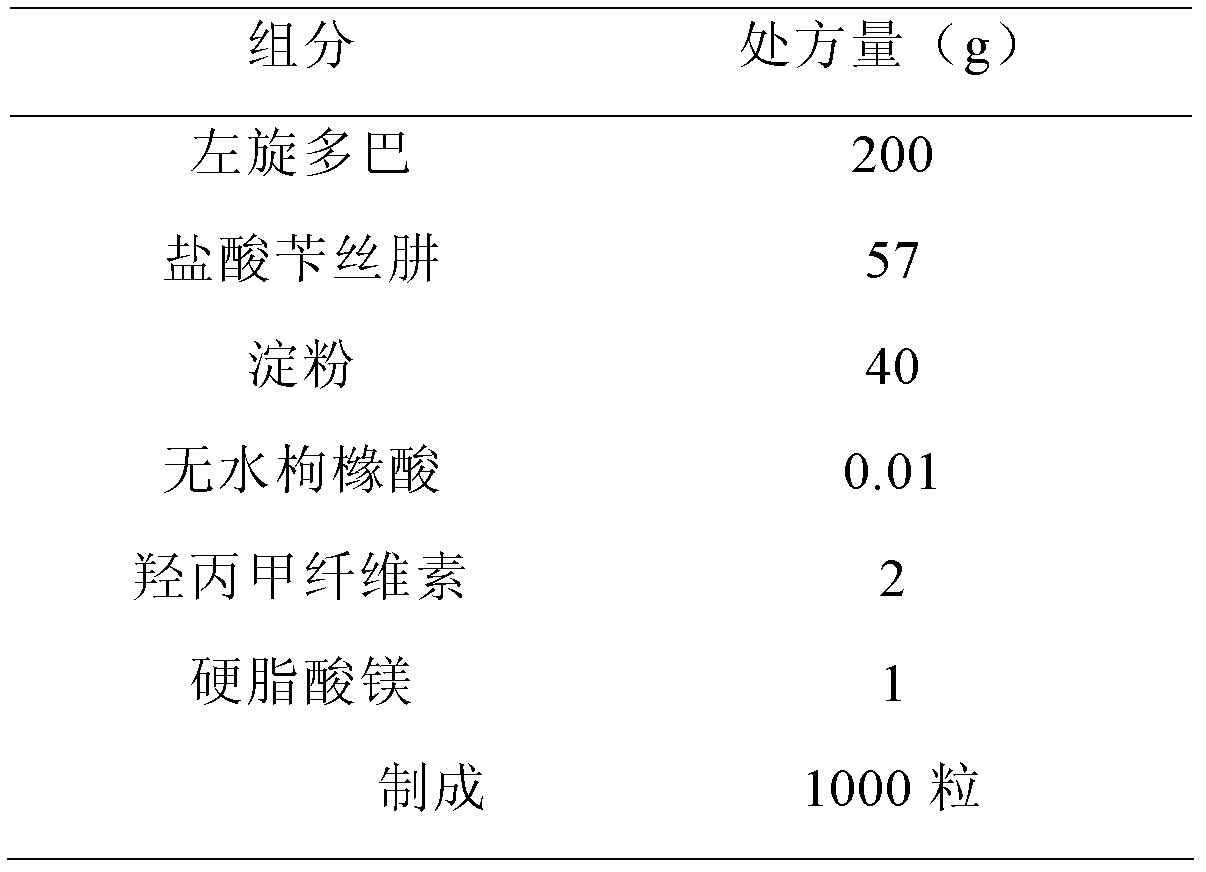
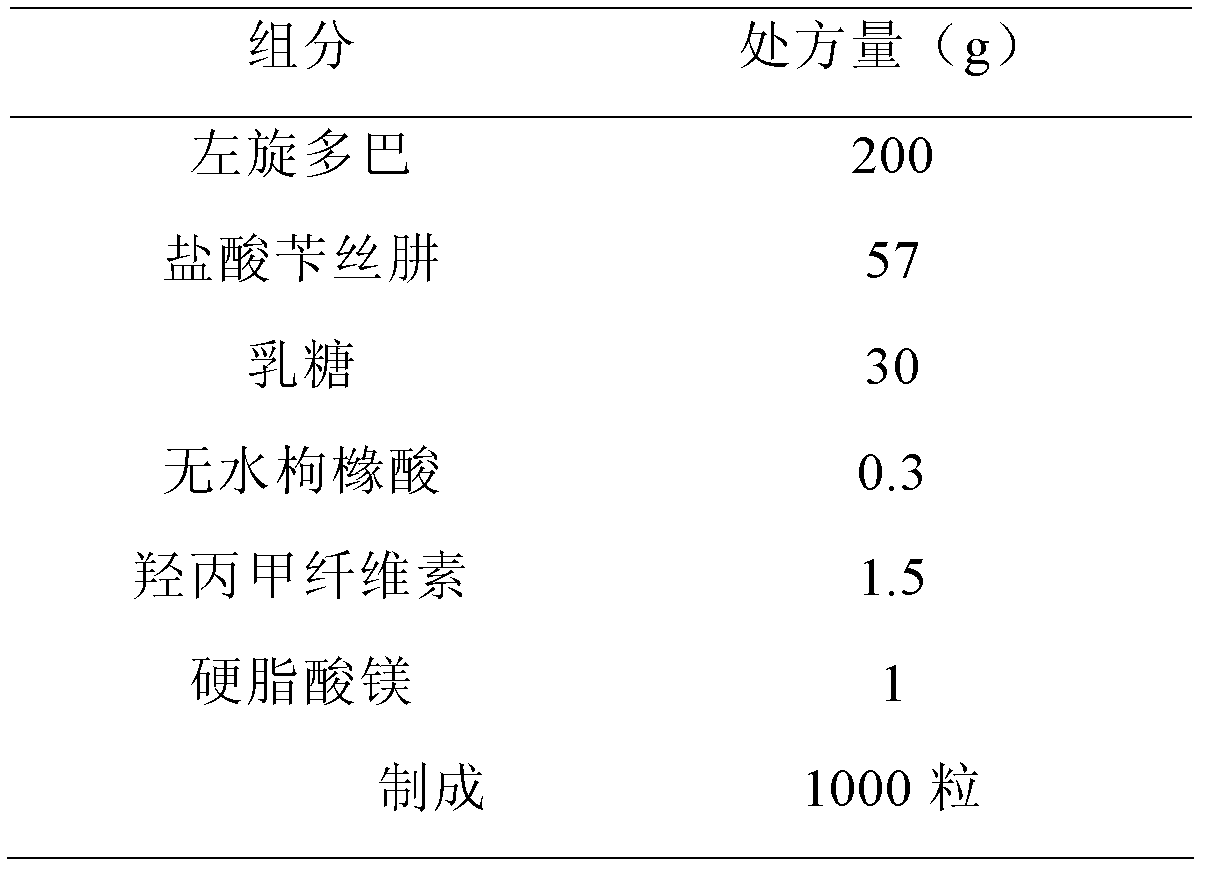
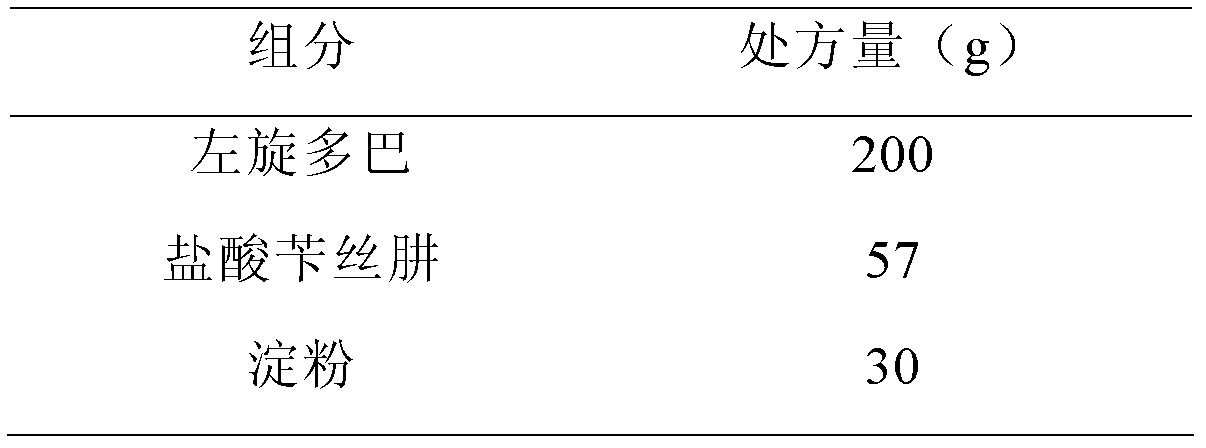
Dopamine prodrug composition and preparation method thereof
ActiveCN102885804AGood stabilitySimple recipeOrganic active ingredientsNervous disorderQuality standardAdhesive
The invention relates to a dopamine prodrug composition and a preparation method of the dopamine prodrug composition. The invention provides a pharmaceutical composition of dopamine prodrug, and the composition comprises the dopamine prodrug, benserazide hydrochloride, an adhesive, a stabilizer and a pharmaceutically acceptable carrier. The pharmaceutical composition containing the dopamine prodrug provided by the invention has excellent stability and does not occur color change, and is capable of effectively guaranteeing that the product quality meets a quality standard requirement within a storage period. The pharmaceutical composition containing the dopamine prodrug provided by the invention has a simple prescription, and auxiliary materials are cheap and available with a small amount; the preparation process provided by the invention is simple and easy to control, used devices are the most conventional workshop production devices, the cost is low, and the preparation process is suitable for industrial production.
Owner:SHANGHAI SINE PHARMA LAB
Method for preparing dry products of sheep placentas and fetal lambs
ActiveCN101618048AKeep shapeMaintain propertiesMammal material medical ingredientsFood preparationAnimal sciencePre treatment
The invention discloses a method for preparing dry products of sheep placentas and fetal lambs, which comprises the steps of: pretreating a sheep placenta or a fetal lamb; and drying the pretreated sheep placenta or fetal lamb. The method is characterized in that the drying is microwave vacuum drying. The prepared sheep placenta or fetal lamb is integrally treated to ensure that original shapes and characteristics of the sheep placenta and the fetal lamb are retained; advanced low-temperature composite sterilization technology is adopted to ensure the safety of a product; the low-temperature microwave vacuum drying is adopted, the sheep placenta has over 95 percent of effective components, and the product has good water reabsorbing capacity; the method has the advantages of simple process, low cost and the like; and the product can be stored and transported for a long time, and can greatly improve the utilization rate of the sheep placenta and the fetal lamb. The product has the advantages of steady quality, high safety and higher commercial value.
Owner:GOLDSUN TRADING SWITZERLAND +1
Extraction method of silibinin
The invention discloses an extraction method of silibinin. The extraction method includes following steps: (1), washing silibinin to be extracted, draining water, and performing microwave drying to enable moisture in silibinin to be lowered to below 10%; (2), adding ethyl acetate to dissolve silibinin, extracting total flavone, and collecting ketone in vacuum; (3), adding water to dissolve total flavones to obtain precipitate, adding alcohol solution for dissolving to obtain a silibinin coarse product, adopting acetone as a solvent, adding the silibinin coarse product, heating for dissolving,and heating till backflow; (4), performing acid pickling on saturated macroporous resin and alcohol washing sequentially to obtain alcohol solution of silibinin; (5), cooling and stirring for crystallization, stirring, filtering, washing with 95% alcohol, draining, and drying a filter cake to obtain a silibinin finished product; (6), performing secondary vacuum drying and concentrating to obtain refined silibinin. The extraction method is simple, efficient, free of harmful and toxic solvent residue, high in product purity, environment-friendly and suitable for industrial production.
Owner:江苏健佳药业有限公司
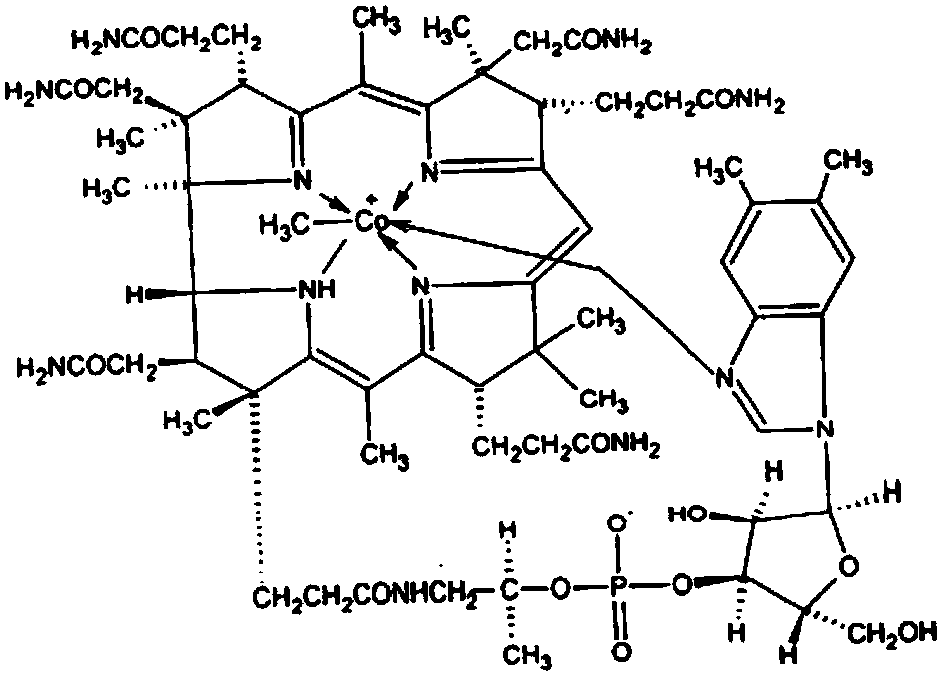
Method used for preparing mecobalamin
ActiveCN107698642AShort reaction timeThe methylation reaction is completeSugar derivativesSugar derivatives preparationButanoneSodium borohydride
The invention discloses a method used for preparing mecobalamin. The method comprises: dissolving cyanocobalamin, butanone and cobalt chloride hexahydrate in purified water, adding a sodium borohydride solution drop by drop for a reduction reaction, adding trimethylsulfoxonium iodide drop by drop for a methylation reaction to generate mecobalamin, and refining mecobalamin through a crystallizationmethod to obtain high-purity mecobalamin. The provided method is simple and easy, is simple to operate, complete in reaction, mild in condition, high in yield and high in purity, and is particularlysuitable for massive production.
Owner:广州普星药业有限公司
A method for synthesizing pitavastatin tert-butyl ester
ActiveCN111875538BHigh stereoselectivityHigh purityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystQuinoline
This invention discloses a method for synthesizing pitavastatin tert-butyl ester, comprising the following steps: (4R-Cis)-6-chloromethyl-2,2-dimethyl-1,3-dioxolane-4-acetic acid tert-butyl ester reacts with substance A under the action of a first base catalyst to obtain substance B; then, it is oxidized by an oxidant to obtain substance C; then, it reacts with 2-cyclopropyl-4-(4-fluorophenyl)-quinoline-3-carboxaldehyde under the action of a second base catalyst to obtain substance D; finally, it is deprotected by acid to obtain pitavastatin tert-butyl ester. The reaction conditions of this invention are mild and controllable, the reaction conditions for the synthesis of Jurlique olefins do not require ultra-low temperature reactions, the operation is convenient and simple, the stereoselectivity is good, the yield is high, and the synthesized pitavastatin tert-butyl ester is completely free of cis isomers and has high purity.
Owner:CHUZHOU QINGYUN PHARM CO LTD
Method for synthesizing trifluoroiodomethane and pentafluoroethyliodide meanwhile
ActiveCN101219925BConvenient sourceImprove stabilityHalogenated hydrocarbon preparationChemical synthesisAlkaline earth metal
The invention relates to 'a method for synthesizing trifluoroiodomethane and pentafluoraethyl iodide simultaneously,' which belongs to the filed of organic chemical synthesis. The method of synthesizing trifluoroiodomethane and pentafluoraethyl iodide simultaneously is characterized in that a alkali metal salt, a alkali earth metal salt and / or a copper salt are taken as the catalysts to be attached to a porous carrier; oxygen or the mixed gas of the oxygen and inert gas is pumped; a CHF3 and / or a HFC_125 are taken as raw materials; an iodine is taken as an iodinating agent; then iodination occurs in a reactor under high temperature to generate the C2F5I and the CF3I. The porous carrier is a fluoride or an oxyfluoride. The raw material of the invention is nontoxic and is convenient to get;the catalyst does not react with a reaction medium and has good stability. The synthesis process is safe and suitable for technical production.
Owner:泉州宇极新材料科技有限公司
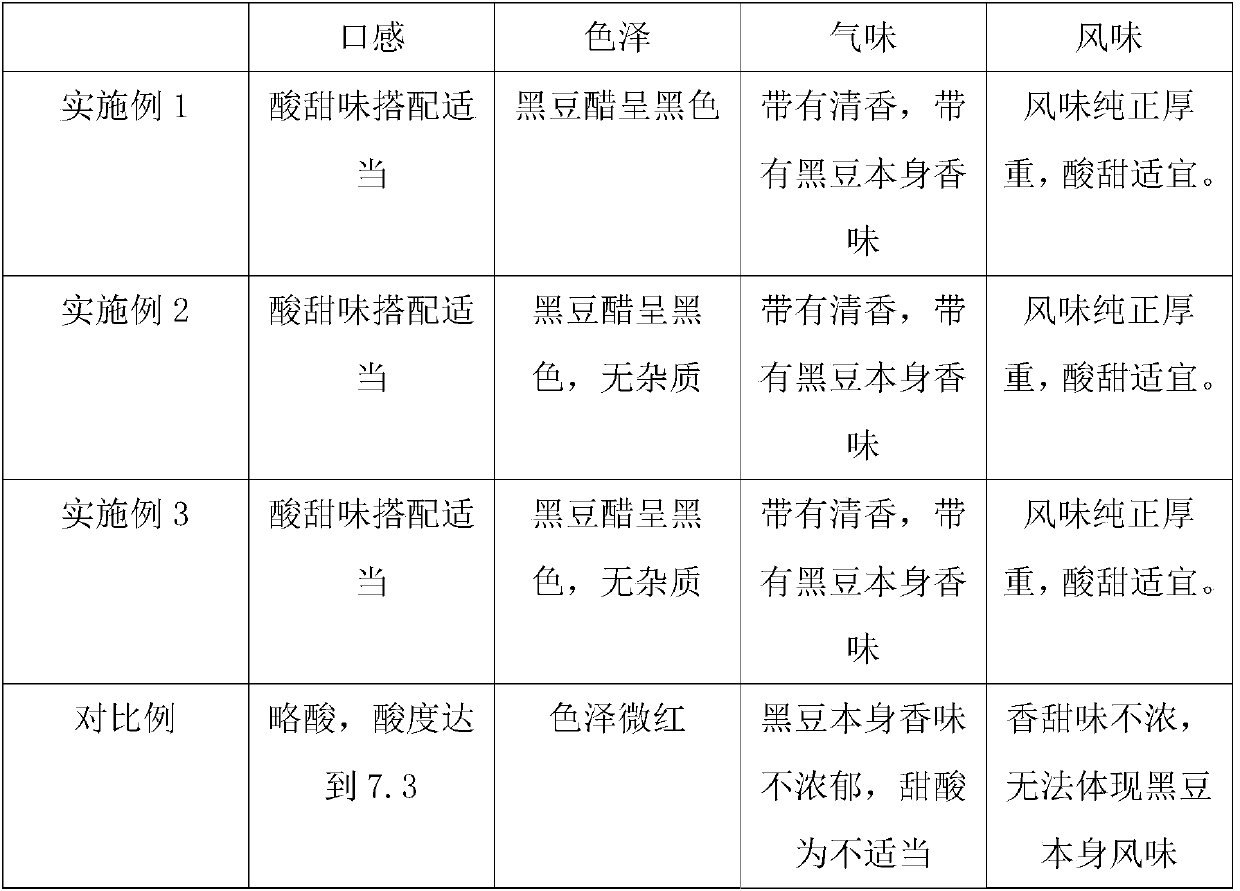
Production technology of black soybean vinegar
InactiveCN107904124ASolve problems that cannot be fully releasedPromote dissolutionVinegar preparationYeastDissolution
The invention belongs to the technical field of food processing and in particular relates to a production technology of black soybean vinegar. The production technology comprises the following steps:firstly, weighing a black soybean mixture, wheat, sorghum rice, peas, smashed bran, cavings and yeast for making hard liquor in proportion; secondly, putting black soybeans, the wheat, the sorghum rice, the peas, the smashed bran and the cavings into a steamer, and steaming for 120 minutes; thirdly, putting cooked raw materials into a fermentation tank, spreading out and cooling, then pouring intothe yeast for making the hard liquor and stirring, and then carrying out constant temperature fermentation for 15 days; fourthly, putting the fermented raw materials into a smoke baking tank, and carrying out smoke baking for 15 days; and fifthly, pouring vinegar on the raw materials subjected to the smoke baking, and then carrying out grooving and grouting conservation or bottling by virtue of apipeline. The production technology provided by the invention has the advantages that the problem that the black soybeans can not be fully released in the prior art is solved, and a black soybean vinegar soaking technology can be effectively replaced, so that dissolution of nutrient substances of the black soybeans can be effectively enhanced, and taste and flavor are also improved.
Owner:靖江市圣材食品有限公司
Method for synthesizing 1,1,2,2-tetrafluoroethane
ActiveCN101591218BConvenient sourceImprove stabilityCatalyst carriersHalogenated hydrocarbon preparationHydrogen fluorideHydrogen
The invention relates to a method for synthesizing 1,1,2,2-tetrafluoroethane, which belongs to the field of preparation of refrigerants. TFE-containing mixed gas and hydrogen pass through a porous carrier supported hydrogenation catalyst bed to generate HFC-134; the TFE-containing mixed gas is a mixture formed by TFE and inert gas; the inert gas is gas which does not react with the TFE and the hydrogen under reaction conditions; and a porous carrier material is fluoride or oxyfluoride. An inert component in the method does not participate in the reaction, but can take away the heat generated by the reaction to ensure that the temperature of the catalyst bed is relatively steady and the service life of a catalyst is prolonged; and the adopted porous carrier is the fluoride or the oxyfluoride, does not react with hydrogen fluoride removed during the reaction, improves the service life of the catalyst and is easy to recycle at the same time. The synthesis method has good stability and safe synthesis process, and is suitable for technologized production.
Owner:泉州宇极新材料科技有限公司
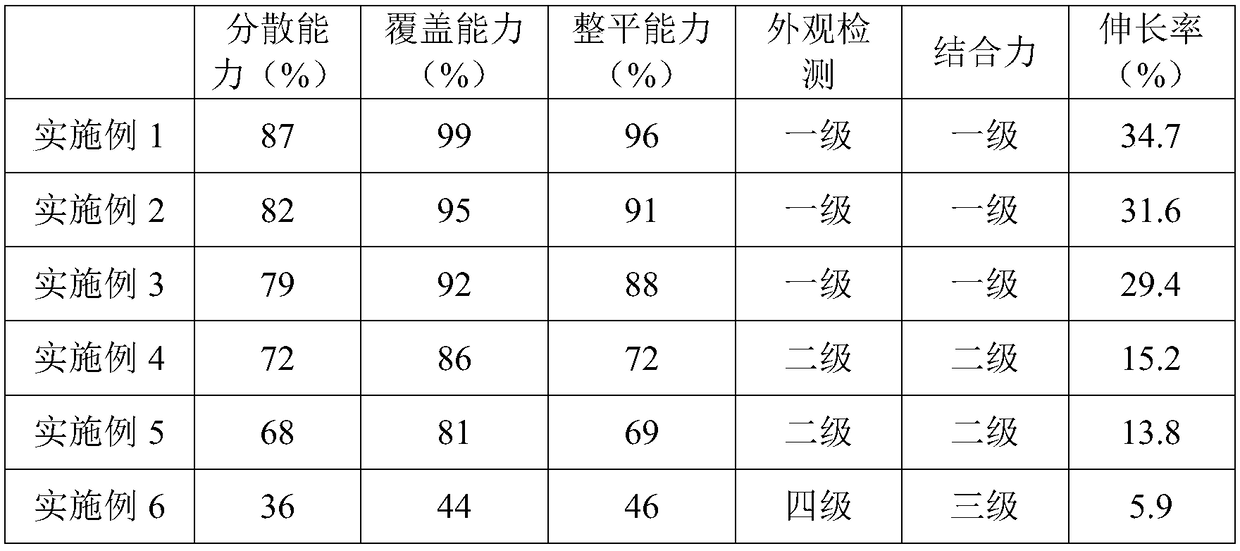
Safe and environment-friendly additive for copper plating, and preparation method of additive
The invention relates to the field of electroplating, in particular to a safe and environment-friendly additive for copper plating, and a preparation method of the additive. The safe and environment-friendly additive, provided by the invention, for copper plating is prepared from 2-8g / L of fatty amine polyoxyethylene ether, 1-5g / L of heterocyclic compound containing sulfhydryl, 5-20g / L of polyethylene imine alkyl compound and 0.5-3g / L of a complexing agent, wherein the total amine value of fatty amine polyoxyethylene ether is 50-60mgKOH / g, and the polyethylene imine alkyl compound contains a heterocyclic ring group.
Owner:上海赛夫特半导体材料有限公司
High-capacity pulse-activating injection and preparation method thereof
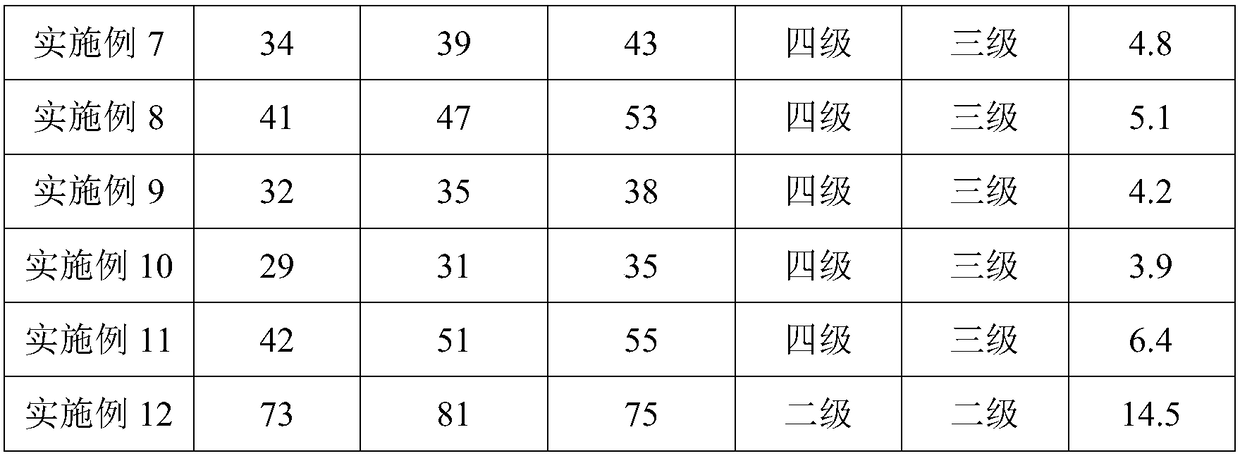
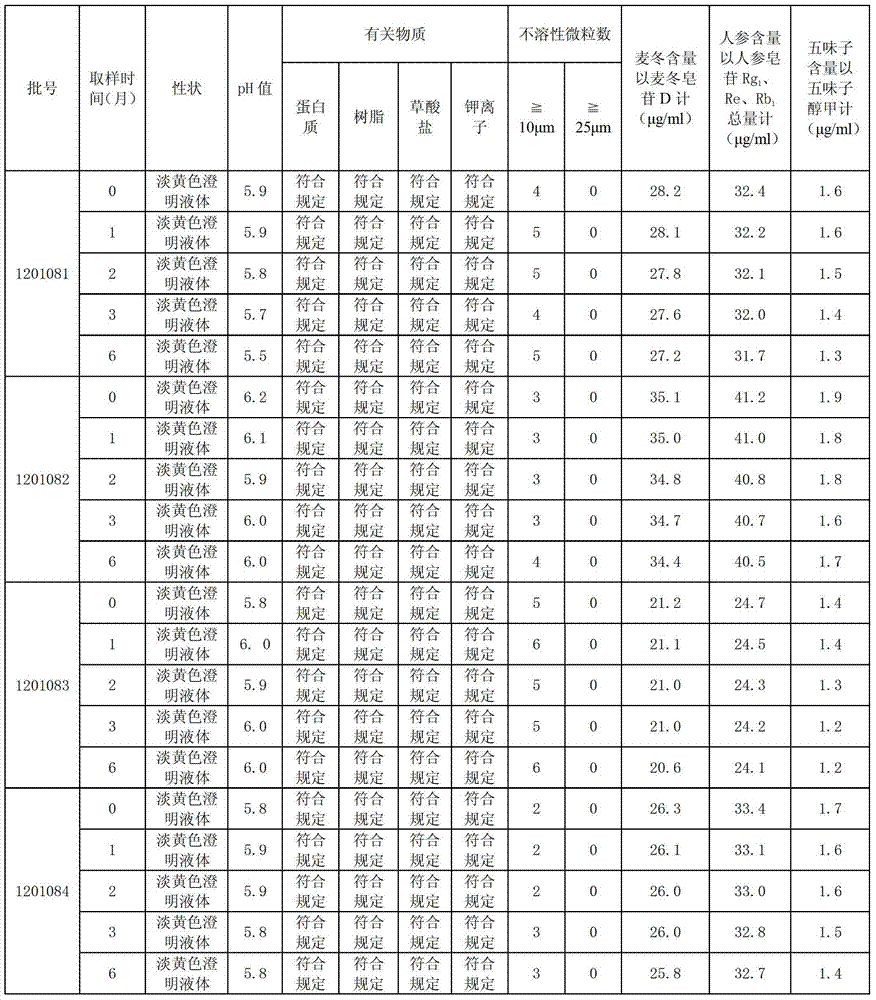
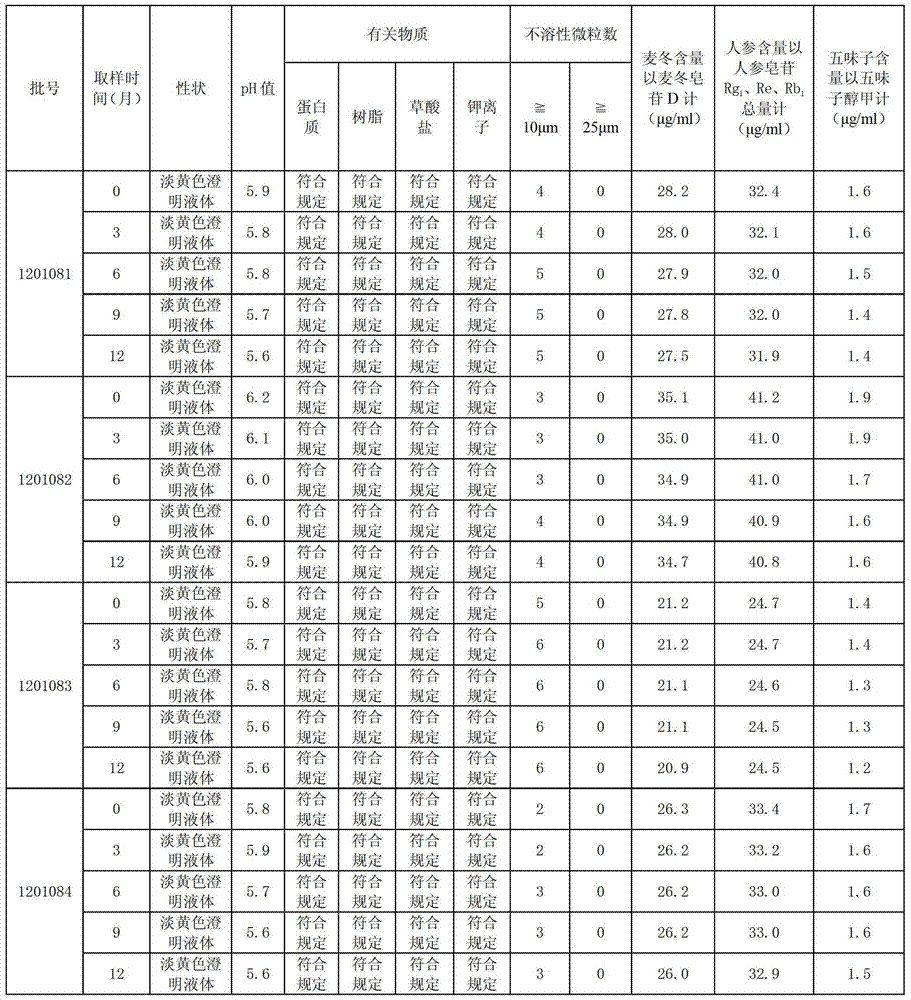
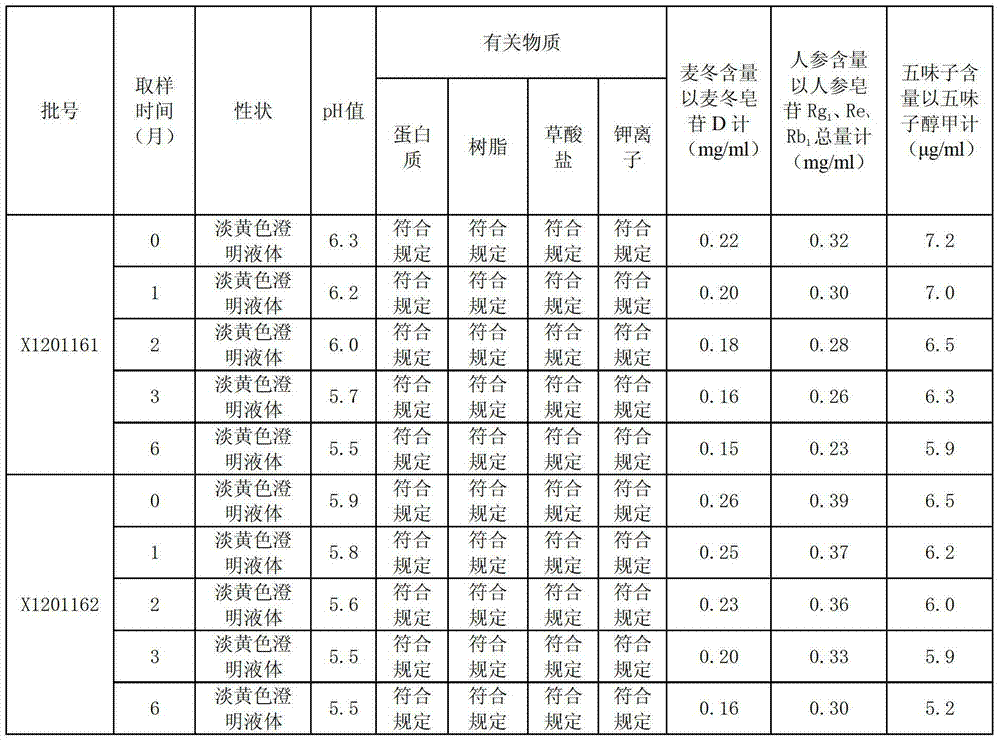
ActiveCN103393928ALess preventionEasy to solvePharmaceutical delivery mechanismAntineoplastic agentsChemistrySchisandra chinensis
The invention discloses a high-capacity pulse-activating injection and a preparation method thereof. The preparation method comprises the following steps of with red ginseng, radix ophiopogonis and schisandra chinensis as raw materials, preparing by using a modern extracting and purifying method to obtain an active part with high effective ingredient content and low impurity component content; then, adding glucose or sodium chloride; and diluting the active part into the injection with the specification of 100-500ml by using water for injection. The high-capacity pulse-activating injection provided by the invention can be directly used for infusion, can be used for avoiding secondary pollution during liquid preparation, and is particularly good in stability and high in safety performance in clinic application; and the preparation method is reasonable in process design and suitable for large-scale industrial production.
Owner:常熟雷允上制药有限公司
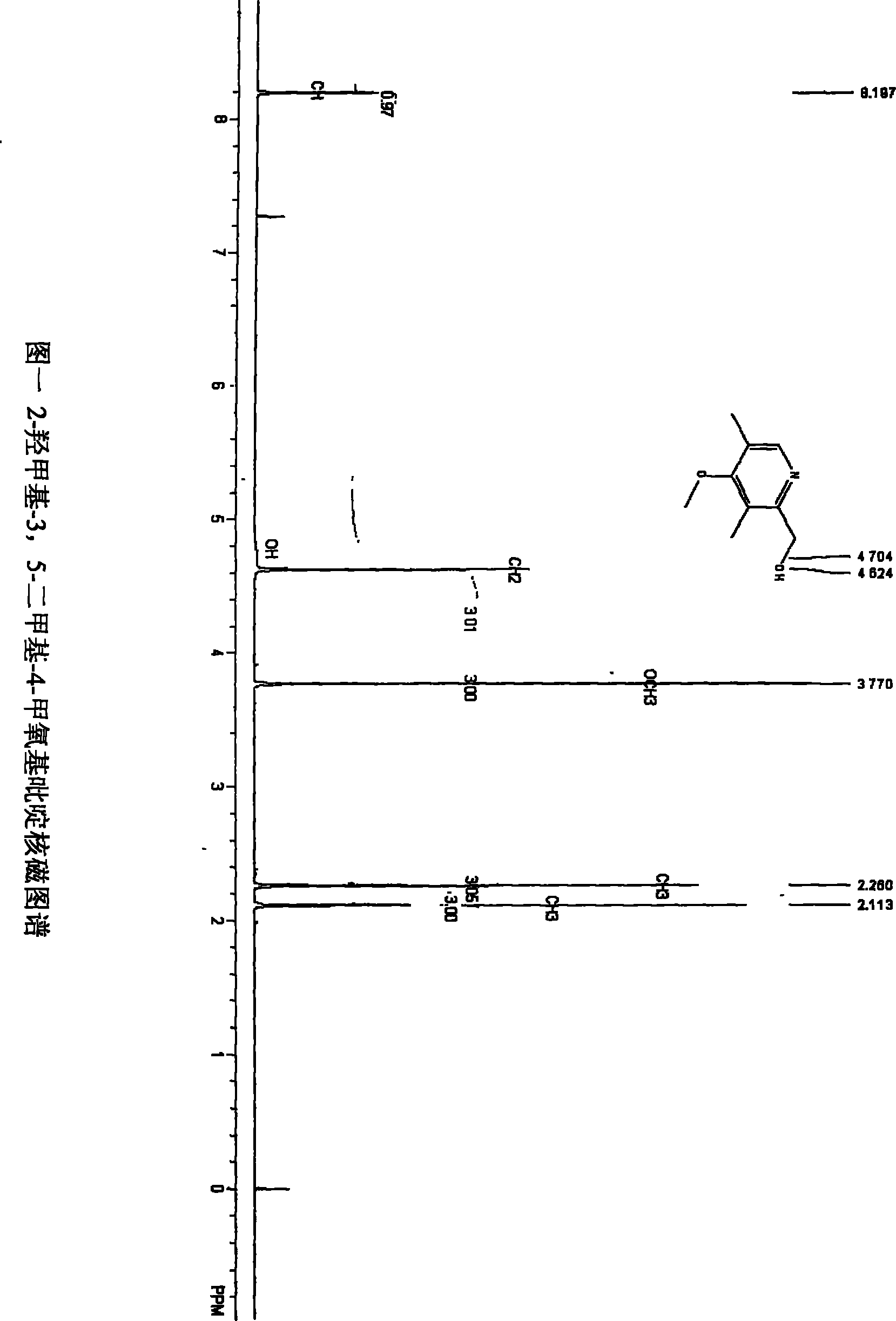
Process for the separation of 2-hydroxymethyl-3, 5-dimethyl-4-methoxy pyridine
ActiveCN101318928BReduce pollutionEasy to operateOrganic chemistryManufacturing cost reduction4-methoxypyridine
The invention relates to a method for separating an intermediate of omeprazole, namely 2-hydroxymethyl-3, 5-dimethyl-4-methoxy pyridine. The method comprises the following steps of: distilling and removing high polymer impurities from a 2-hydroxymethyl-3, 5-dimethyl-4-methoxy pyridine mother solution containing pyridine derivative, high polymer and other impurities by an evaporator, and then separating and removing the pyridine derivative impurities from the mixed solution through a rectifying tower to separate a product. The product obtained by the method has the advantages of high purity, simple and convenient separation method, manufacturing cost reduction, environmental pollution reduction and suitability for industrial production.
Owner:ZHEJIANG HUAYI PHARMA CO LTD OF HANGZHOU HUADONG PHARMA GRP +1
New crystal of rivaroxaban and preparation method thereof
ActiveCN106008490AHigh purityFast dissolutionOrganic active ingredientsOrganic chemistry methodsSolubilityRivaroxaban
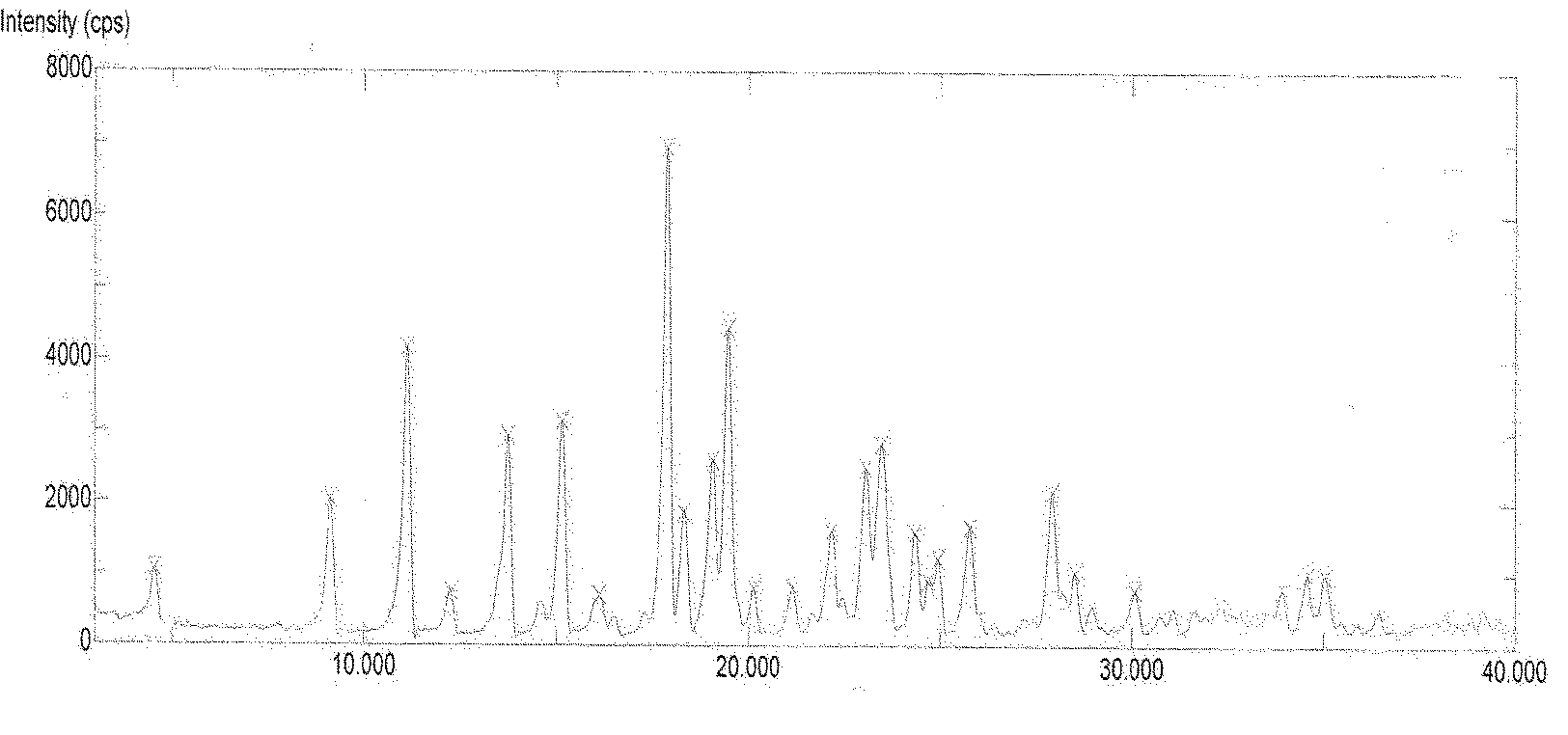
The invention discloses a new crystal of rivaroxaban and a preparation method thereof. The new crystal of rivaroxaban is crystal SMN-F, X-ray powder diffraction spectrum of which has diffraction peaks when a diffraction angle 2theta is 3.7+ / -0.2, 7.3+ / -0.2, 14.5+ / -0.2, 21.8+ / -0.2, 29.2+ / -0.2, 31.9+ / -0.2 and 33.5+ / -0.2. The preparation method of the rivaroxaban SMN-F crystal provided by the invention has the advantages of simple process, short time, and suitability for technological production, etc. At the same time, the rivaroxaban SMN-F crystal prepared by the method provided by the invention has the advantages of high purity, fast dissolution rate and good solubility, etc.
Owner:NANJING LIFENERGY R & D +1
Bee venom peptide and separation and purification method thereof
ActiveCN114773446AReduce lossesReduce dosagePeptide preparation methodsAnimals/human peptidesOrganic solventIsocratic elution
The invention discloses melittin and a separation and purification method thereof, and relates to the technical field of biology. The method comprises the following steps: carrying out primary reversed-phase C18 semi-preparative column separation on a bee venom crude extract to be separated and purified, carrying out gradient elution by taking a formic acid aqueous solution and acetonitrile as mobile phases, collecting an eluent, then freeze-drying the eluent, redissolving, and carrying out secondary reversed-phase C18 semi-preparative column separation on the redissolved solution, in the secondary reversed-phase C18 semi-preparative column separation, a trifluoroacetic acid aqueous solution and methanol are used as mobile phases for isocratic elution. The method can achieve the technical effects of high separation purity, less target substance loss and less organic solvent dosage without combining with an ultrafiltration process for impurity removal in a specific pH environment, and has the technical advantages of environmental friendliness, simple device, simplicity and convenience in process operation and suitability for industrial production.
Owner:INST OF QUALITY STANDARD & TESTING TECH FOR AGRO PROD OF CAAS
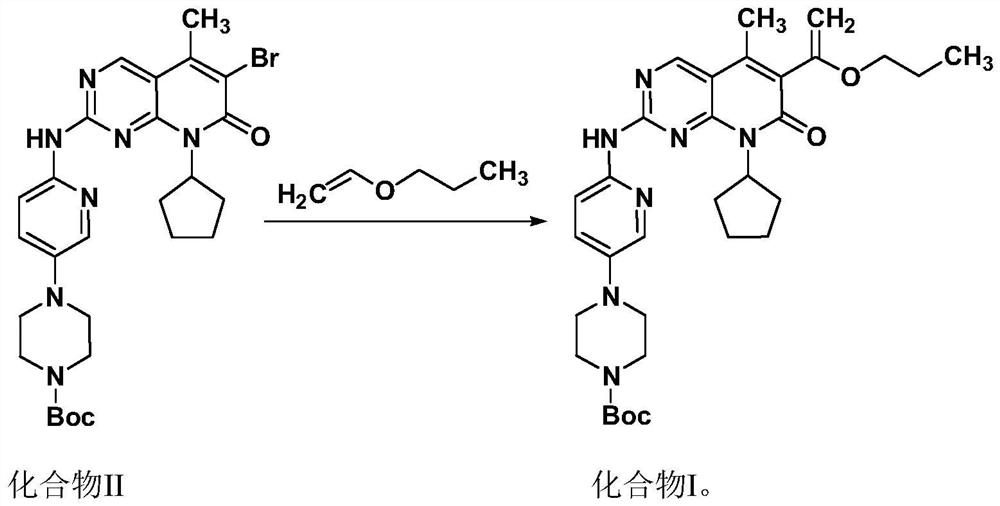
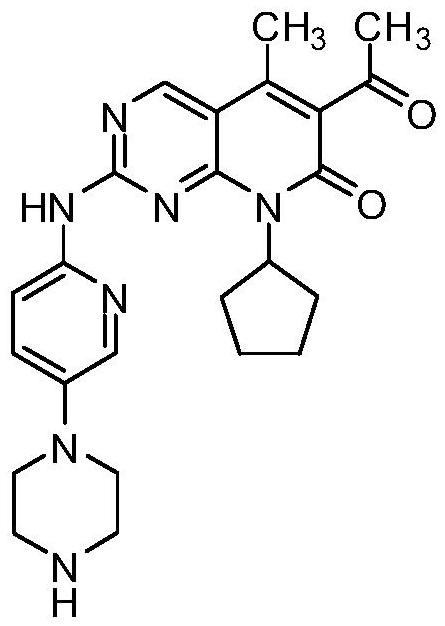
Novel intermediate of palbociclib, and crystal form and preparation method of novel intermediate
InactiveCN111892590ALow costEasy to crystallize and good stabilityOrganic chemistry methodsPyrimidinePalbociclib
The invention belongs to the technical field of medicinal chemistry, and particularly relates to a novel intermediate of palbociclib, namely 4-(6-(8-cyclopentyl-5-methyl-7-oxo-6-(1-propoxyvinyl)-7,8-dihydropyrido[2, 3-d]pyrimidin-2-ylamino)pyridin-3-yl)-piperazinyl-1-carboxylic acid tert-butyl ester, and a crystal form and a preparation method of the novel intermediate. The method is simple in technological operation and post-treatment, a crystallization solvent is easy to recycle, cost is low, and the obtained intermediate is easy to crystallize, good in stability, good in purity, high in yield and more suitable for industrial production.
Owner:QILU PHARMA
Sheep embryo gelatin health food and preparation method thereof
The invention provides a sheep embryo gelatin health food comprising the following constituents of: animal skin gelatin, gelatin, a sheep embryo enzyme hydrolysate, edible sugar, yellow wine and edible vegetable oil, wherein the weight ratio of the animal skin gelatin to the gelatin to the sheep embryo enzyme hydrolysate is (8-12):(2-6):1, the content of amino acid nitrogen in the sheep embryo enzyme hydrolysate is (2-20)g / 100ml, and the intrinsic viscosity Eta of the animal skin gelatin is (10-6)dL / g. The obtained health food contains abundant amino acids and some elements which facilitate the absorption by the body and are good for human health, can be used for regulating the body balance better, improving the circulation and preventing the arteriosclerosis, has a high effect of beauty and curative effects on a coronary heart disease and an ischemic brain disease, and also is suitable for critically ill patients such as a diabetic patient, a kidney patient and the like to take a high-quality high-protein health food.
Owner:GOLDSUN TRADING SWITZERLAND +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com