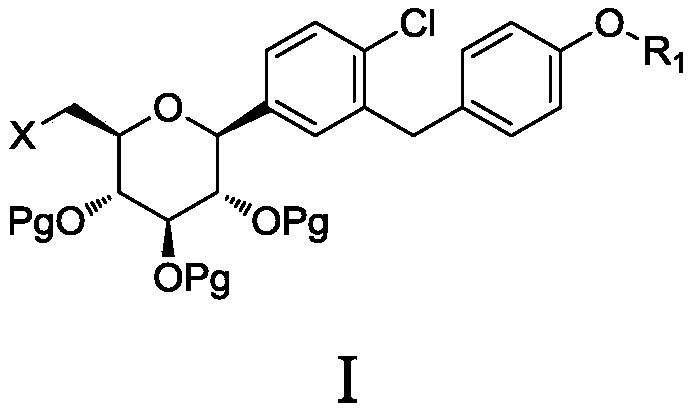
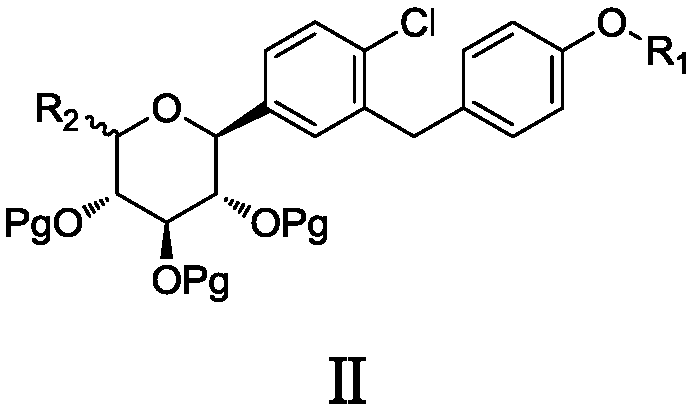
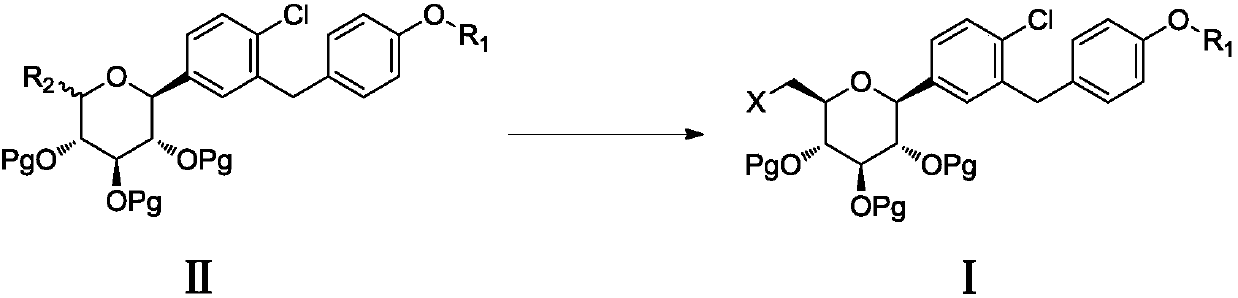
6-halogenated glucose C-glycoside as well as preparation method and application thereof
A technology of glucose and carbon glycosides, applied in 6-halogenated glucose carbon glycosides and its preparation, preparation of SGLT inhibitor-type diabetes drug intermediates and preparation fields, can solve the problem of poor stability, poor selectivity, and increase of epoxide intermediates. problems such as the quality risk of large raw materials, to achieve the effect of low cost, low price and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1: (3aS,5R,6S,6aS)-6-hydroxy-2,2-dimethyltetrahydrofuro[2,3-d][1,3]dioxolane-5-carboxylic acid (compound A) Synthesis
[0063]
[0064] Synthesis of (3aS,5R,6S,6aS)-5-hydroxymethyl-2,2-dimethyltetrahydrofuro[2,3-d][1,3]dioxolane-6-ol
[0065] To a 3 L three-necked flask was added L-(-)-xylose (100.00 g, 0.67 mol), acetone (1 L) and anhydrous magnesium sulfate (160.00 g, 1.32 mol). The obtained suspension was stirred at room temperature for 10 to 15 minutes, and then 10 mL of concentrated sulfuric acid (0.19 mol) was slowly added dropwise. The reaction was continued to stir overnight at room temperature. After TLC detected the reaction, the reaction mixture was filtered, and the filter cake was washed twice with 100 mL×2 acetone. The obtained filtrate was adjusted to pH 8-9 with ammonia water, and after stirring for 30 minutes, the suspension was filtered. The filtrate was concentrated to dryness under reduced pressure at 35-45°C to obtain 143.89 g of a yel...
Embodiment 2
[0069] Example 2: ((3aS,5R,6S,6aS)-6-hydroxy-2,2-dimethyltetrahydrofuro[2,3-d][1,3]dioxolane-5-)( Synthesis of morpholino)methanone (compound B)
[0070]
[0071] The above-obtained acid A (99.9g, 0.49mol) was added to a 3L three-necked flask, 1L of tetrahydrofuran was added, stirred to dissolve, and TBTU (235.76g, 0.74mol) and N-methylmorpholine (NMM, 82mL) were added successively. 0.74mol). The resulting reaction mixture was stirred at room temperature overnight. The reaction solution was filtered, and the filter cake was washed with 200 mL×2 tetrahydrofuran. The filtrate was concentrated to dryness under reduced pressure at 35-45 °C, 400 mL of hexane was added to the obtained slurry, heated to 60-70 °C, stirred for 1 hour, cooled to room temperature for 2 hours, and then cooled to 0-10 °C for 2 hours. After 1 hour, 111.02 g of white solid was obtained by filtration, and the yield was 82.6%. ESI-MS: m / z 274[M+H] + .
Embodiment 3
[0072] Example 3: Synthesis of 1-chloro-2-(4-ethoxybenzyl)-4-bromobenzene
[0073]
[0074] Into a 500 mL three-necked round bottom flask, were added 5-bromo-2-chlorobenzoic acid (20 g, 84.9 mmol), dichloromethane (100 mL), and N,N-dimethylformamide (0.1 mL). After stirring for 10 minutes, oxalyl chloride (13.0 g, 102.7 mmol, 1.2 eq) was added dropwise to the mixture at room temperature. After the dropwise addition, the reaction was stirred at room temperature for 3-4 hours. The reaction solution was concentrated to dryness under reduced pressure at 30-40° C. to obtain an off-white solid, that is, crude 5-bromo-2-chlorobenzoyl chloride. The obtained crude 5-bromo-2-chlorobenzoyl chloride was dissolved in 50 mL of dichloromethane and used for later use.
[0075] Take another 500mL three-necked round bottom flask, add phenethyl ether (13.5g, 110.4mmol, 1.3eq), dichloromethane (100mL), aluminum trichloride (17.0g, 127.4mmol, 1.5eq). The obtained mixed solution is fully stir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com