New crystal of rivaroxaban and preparation method thereof
A technology for rivaroxaban and crystals, applied in the field of new rivaroxaban crystals and their preparation, can solve problems such as solvent residues, and achieve the effects of fast dissolution rate, good solubility and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Add 2 g (purity: 95.0%) of the crude product of rivaroxaban crystal form I to 8 g of formic acid to form a 20% rivaroxaban suspension, heat the suspension to dissolve rivaroxaban, add 40 g of acetonitrile and water 2g, then the solution was cooled to room temperature, solids were precipitated, filtered, the filter cake was placed in a vacuum drying oven, and dried under reduced pressure (vacuum degree of 0.085mPa, drying temperature of 20°C) to constant weight to obtain rivaroxaban crystal SMN -F.
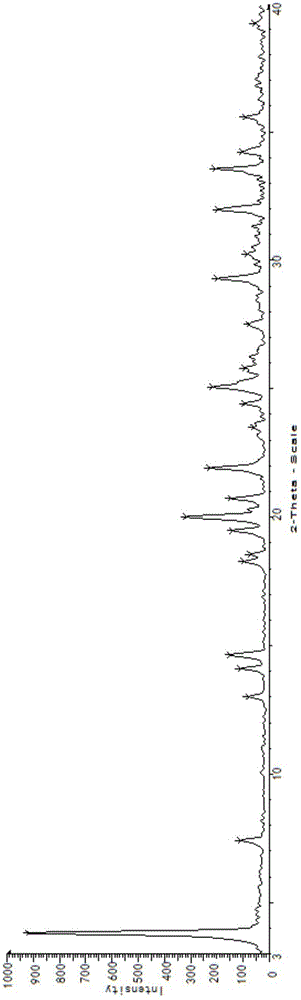
[0043] The X-ray powder diffraction pattern of the crystal that present embodiment makes is 3.7±0.2, 7.3±0.2, 14.0±0.2, 14.5±0.2, 18.2±0.2, 18.4±0.2, 19.9±0.2, 20.6±0.2, Features at 21.8±0.2, 23.1±0.2, 25.0±0.2, 26.4±0.2, 28.4±0.2, 29.2±0.2, 31.9±0.2, 33.5±0.2, 34.1±0.2, 35.5±0.2, 36.8±0.2, 37.1±0.2 There are characteristic peaks at the absorption kurtosis, and its specific spectrum is as follows figure 1 shown.
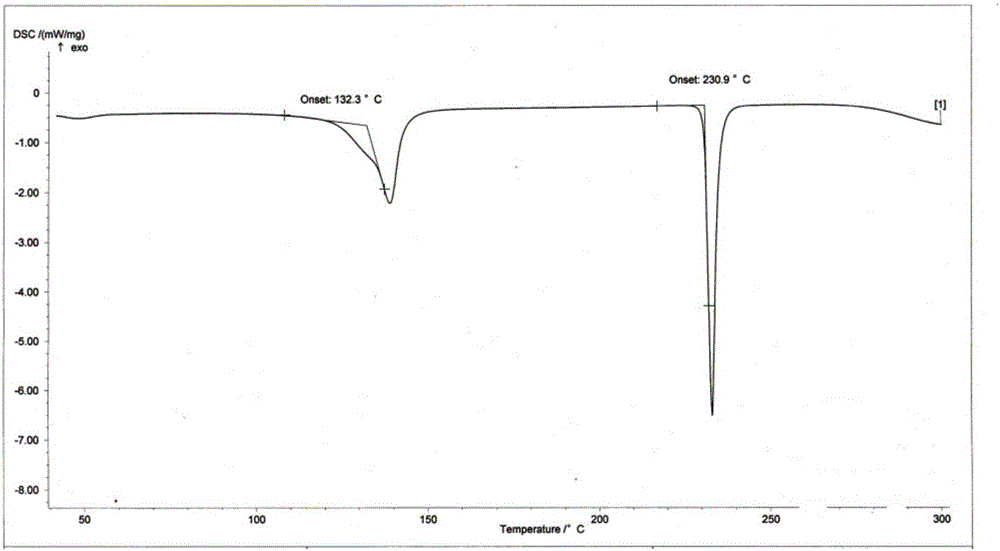
[0044] The DSC collection of illustrative plates of the cr...
Embodiment 2
[0056] Add 2 g of rivaroxaban crystal form II crude product (purity: 97.3%) to 8 g of formic acid to configure a 20% rivaroxaban suspension, heat the suspension to dissolve rivaroxaban, add 60 g of acetonitrile and water 4g, then the solution was cooled to room temperature, solids were precipitated, filtered, the filter cake was placed in a vacuum drying oven, and dried under reduced pressure (vacuum degree of 0.80mPa, drying temperature of 30°C) to constant weight to obtain rivaroxaban crystal SMN -F.
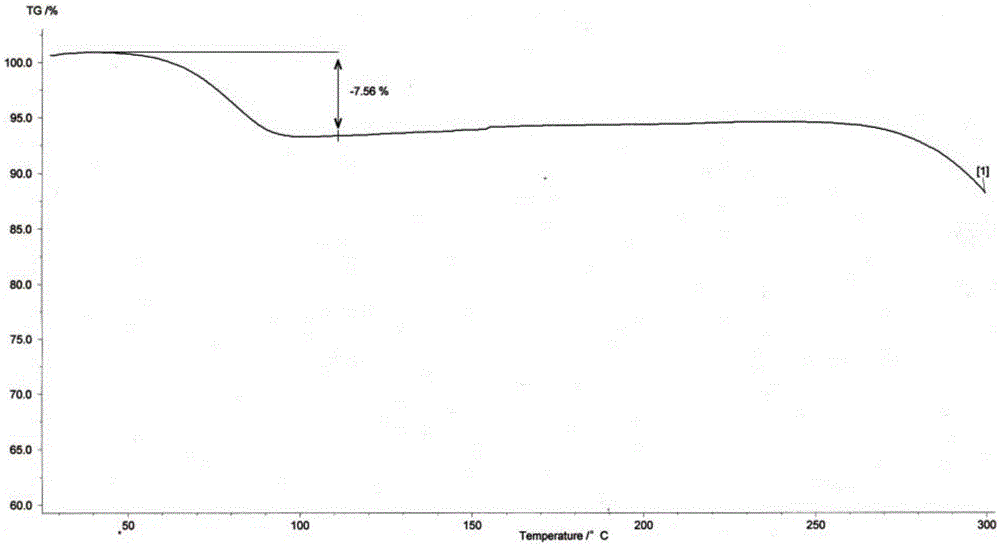
[0057] The crystal product obtained in this embodiment has a purity of 99.91% and a water content of 7.63%. The characteristic data of this embodiment are the same as that of Embodiment 1.
Embodiment 3
[0059] Add 2 g (purity: 95.0%) of rivaroxaban crystal amorphous crude product into 18 g formic acid to form a 10% rivaroxaban suspension, heat the suspension to dissolve rivaroxaban, add 60 g of acetonitrile and 4g of water, then the solution was cooled to room temperature, solids were precipitated, filtered, the filter cake was placed in a decompression drying oven, and dried under reduced pressure (vacuum degree of 0.90mPa, drying temperature of 10°C) to constant weight to obtain rivaroxaban crystals SMN-F.
[0060] The crystal product obtained in this embodiment has a purity of 99.93% and a water content of 7.66%. The characteristic data of this embodiment are the same as that of Embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com