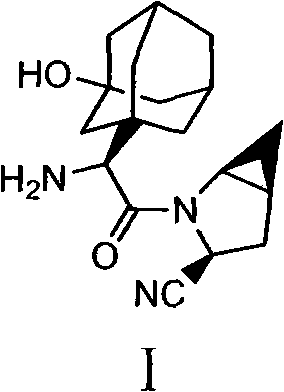
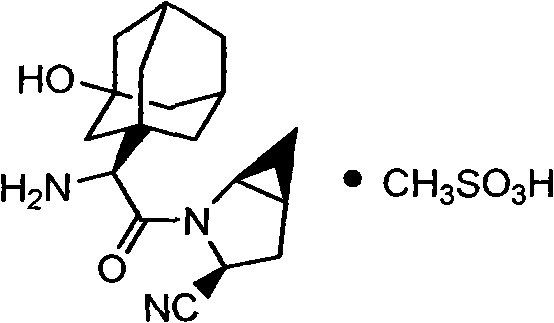
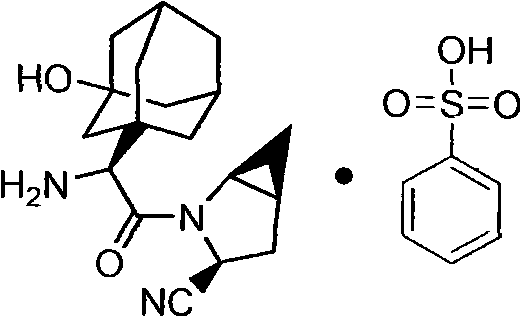
Medicinal salts of saxagliptin and preparation methods of medicinal salts
A technology of medicinal salt and maleate, which is applied in the field of pharmacy, can solve the problems of complex preparation process, difficulty in realizing industrial production, and difficulty in quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] The preparation of embodiment 1 saxagliptin mesylate
[0082] Saxagliptin (0.50g, 1.59mmol) was dissolved in 8ml ethanol solution, stirred, heated to 40°C, slowly added 2ml ethanol containing methanesulfonic acid (0.15g, 1.59mmol), cooled to room temperature after addition, Add 16ml of diethyl ether, continue stirring for 1 hour, cool down to 0°C and stir overnight, filter, and vacuum-dry the filter cake at 30°C to obtain 0.43g of light yellow powdery solid, yield 66.2%.
[0083] 1 HNMR (CD 3 OD), δ(ppm): 0.94(m, 1H), 1.10(m, 1H), 1.52-1.88(m, 12H), 2.02(m, 1H), 2.27(s, 2H), 2.34(m, 1H ), 2.60(m, 1H), 2.89(s, 3H), 3.93(m, 1H), 4.28(s, 1H), 5.20(m, 1H).
[0084] ESI-MS m / z: 316.2[M+H] + .
[0085] Elemental analysis (C 19 h 29 N 3 o 5 S·H 2 O): Tested value (%): C, 53.02; H, 7.35; N, 9.63 (calculated value (%): C, 53.13; H, 7.27; N, 9.78).
Embodiment 2
[0086] The preparation of embodiment 2 saxagliptin maleate (1: 1)
[0087] Saxagliptin (3.50 g, 11.1 mmol) was dissolved in 25 ml of ethyl acetate solution, stirred, heated to 45° C., slowly added in maleic acid (1.29 g, 11.1 mmol) in batches, and stirred for 10 minutes after the addition was completed. Cool down to room temperature, slowly add 25ml of cyclohexane, continue to stir for 1 hour, cool down to 0°C and stir overnight, filter, and vacuum-dry the filter cake at 30°C to obtain 3.46g of white powdery solid with a yield of 72.2%.
[0088] 1 HNMR (CD 3 OD), δ(ppm): 0.95(m, 1H), 1.12(m, 1H), 1.54-1.88(m, 12H), 2.01(m, 1H), 2.27(s, 2H), 2.34(m, 1H ), 2.61 (m, 1H), 3.93 (m, 1H), 4.28 (s, 1H), 5.19 (m, 1H), 6.28 (s, 2H).
[0089] ESI-MS m / z: 316.2[M+H] + .
[0090] Elemental analysis (C 22 h 29 N 3 o 6 ): Tested value (%): C, 61.09; H, 6.81; N, 9.48 (calculated value (%): C, 61.24; H, 6.77; N, 9.74).
Embodiment 3
[0091] The preparation of embodiment 3 saxagliptin maleate (2: 1)
[0092] Saxagliptin (0.50g, 1.59mmol) was dissolved in 5ml of ethyl acetate solution, stirred, heated to 45°C, slowly added in maleic acid (0.09g, 0.78mmol) in batches, and stirred for 10 minutes after the addition was complete. Cool down to room temperature, slowly add 5ml of cyclohexane, continue to stir for 1 hour, cool down to 0°C and stir overnight, filter, and vacuum-dry the filter cake at 30°C to obtain 0.38g of white powdery solid with a yield of 64.4%.
[0093] 1 HNMR (CD 3 OD), δ(ppm): 0.95(m, 2H), 1.10(m, 2H), 1.52-1.86(m, 24H), 2.00(m, 2H), 2.28(s, 4H), 2.34(m, 2H ), 2.61 (m, 2H), 3.93 (m, 2H), 4.28 (s, 2H), 5.19 (m, 2H), 6.30 (s, 2H).
[0094] ESI-MS m / z: 316.2[M+H] + .
[0095] Elemental analysis (C 40 h 54 N 6 o 8 ): Tested value (%): C, 64.47; H, 7.35; N, 11.09 (calculated value (%): C, 64.32; H, 7.29; N, 11.25).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com