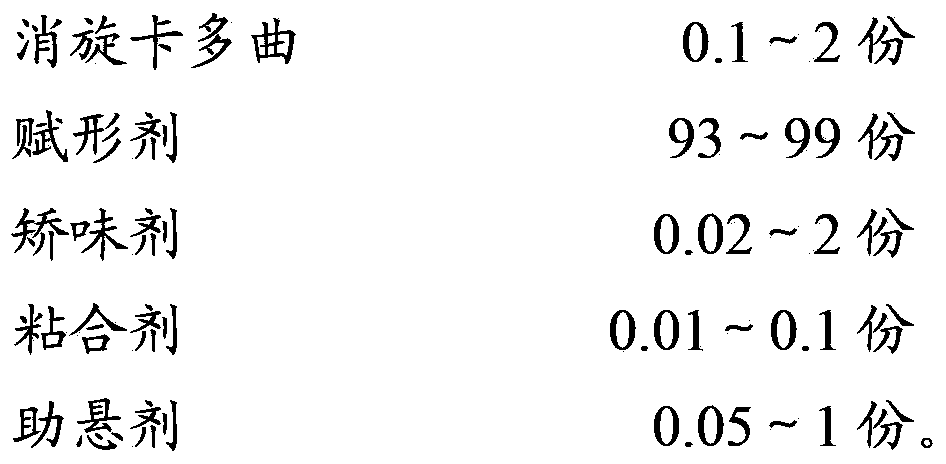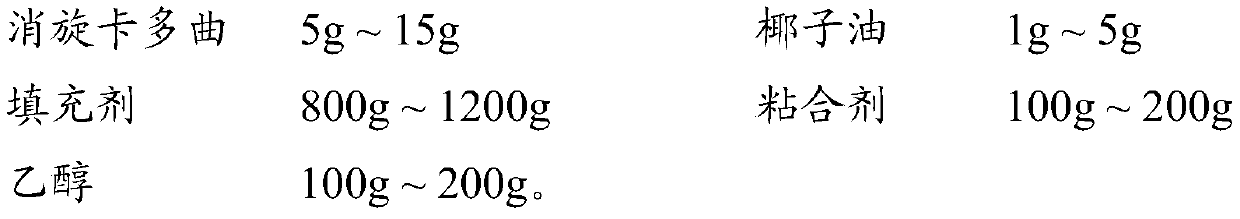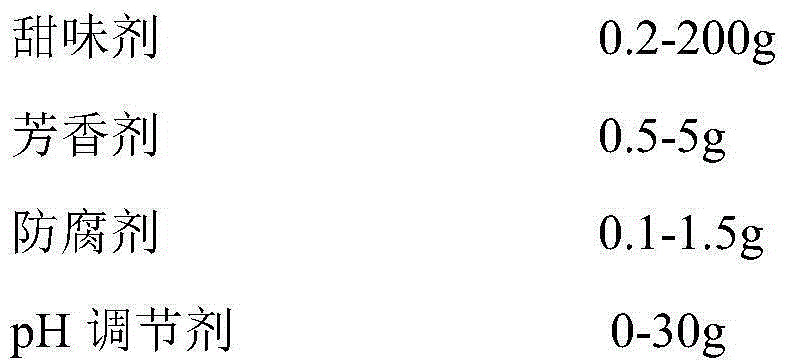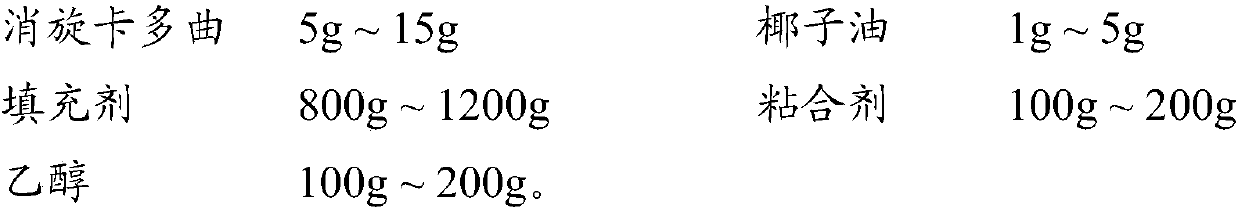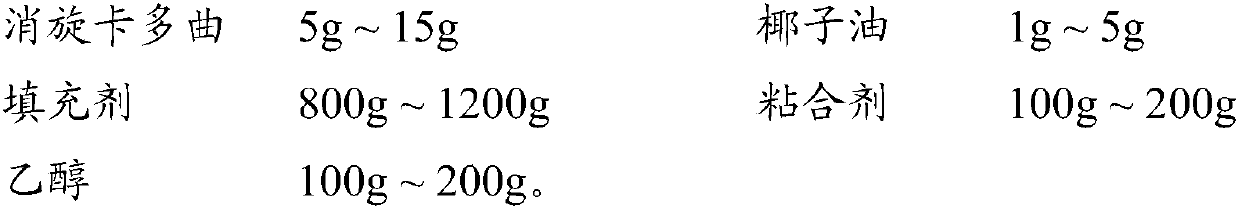Patents
Literature
54 results about "Racecadotril" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
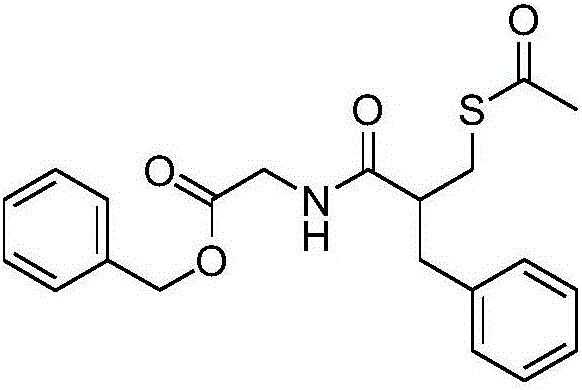
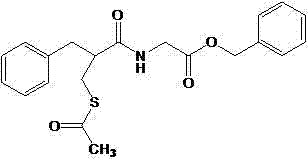
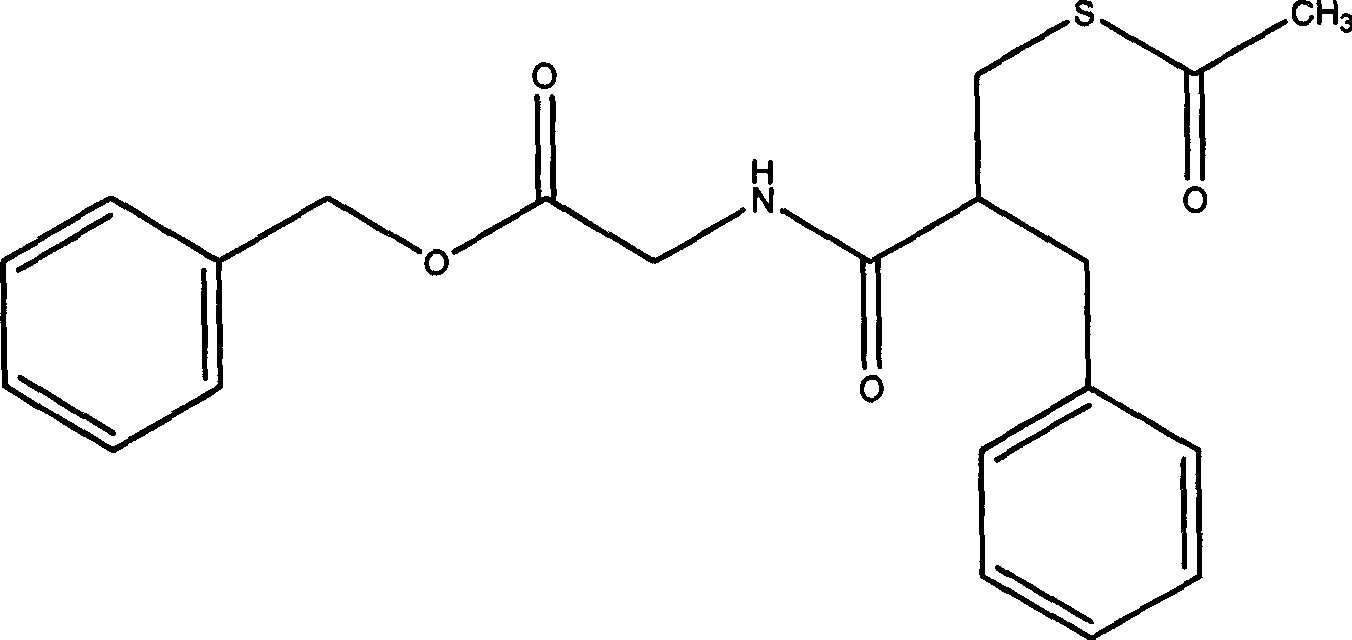
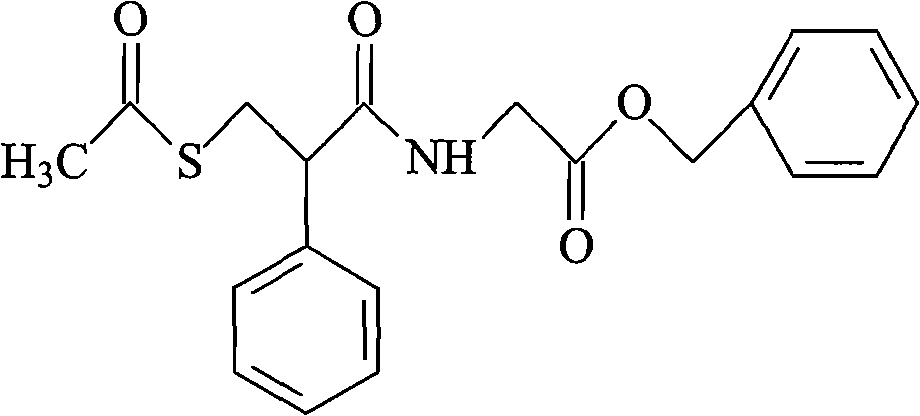
Racecadotril, also known as acetorphan, is an antidiarrheal drug which acts as a peripherally acting enkephalinase inhibitor. Unlike other opioid medications used to treat diarrhea, which reduce intestinal motility, racecadotril has an antisecretory effect—it reduces the secretion of water and electrolytes into the intestine. It is available in France (where it was first introduced in ~1990) and other European countries (including Germany, Italy, the UK, Spain, Russia and the Czech Republic) as well as most of South America and some South East Asian countries (including China, India and Thailand), but not in the United States. It is sold under the tradenames Hidrasec or, in France, Tiorfan. In Italy it is sold under the tradename Tiorfix. In India it's available as Redotril and enuff. Thiorphan is the active metabolite of racecadotril, which exerts the bulk of its inhibitory actions on enkephalinase.
Racecadotril liposome solid preparation
InactiveCN102133186AImprove solubilityFast absorbingOrganic active ingredientsDigestive systemSolubilitySide effect
The invention discloses a racecadotril liposome solid preparation. The racecadotril liposome is prepared from racecadotril, soy lecithin, hydrogenated egg lecithin, cholesterol and tween 80 in the weight ratio of 1:2-8: 2-8:1.5-6:0.5-3; and the solid preparation is prepared from the racecadotril liposome and other pharmaceutically common auxiliary materials. The other pharmaceutically common auxiliary materials comprise filler, a disintegrating agent, a sweetener, an adhesive and a lubricant. The racecadotril liposome solid preparation improves the dissolubility of the racecadotril so as to improve bioavailability; a medicament is absorbed and distributed in a body fast; a curative effect is improved obviously; and the product quality of the preparation is improved and the toxic and side effects are reduced.
Owner:HAINAN MEIDA PHARMA
Racecadotril dropping pill and preparation thereof
InactiveCN101264065AGood dispersionIncrease the effective surface areaOrganic active ingredientsDigestive systemMarked effectTemperature control
The invention provides a racecadotril pill which is a composition used for curing acute diarrhea. The racecadotril pill takes g or Kg as the unit and adopts the weight ratio that racecadotril to polyethylene glycol (4000 to 10000) to polyoxyethylene (40) stearate is equal to 1: 1: 1 to 1: 3: 3. The invention also relates to a preparation method, which means that pro rata matching -> making melt liquid and / or emulsion and / or suspension by heating for standby -> adjusting the temperature control system of pill machine -> dropping pill and forming -> removing condensing agent and drying. The racecadotril pill has the advantages of high bioavailability, rapid releasing speed and rapid marked effect. The preparation method of racecadotril pill has the advantages of simple production technology, low production cost, non-pollution of production process and high yield.
Owner:YANCHENG SUHAI PHARMA
Dry mixed suspension containing racecadotril and preparation method thereof
ActiveCN101103960AGreat tasteSolving the Settling Volume Ratio ProblemOrganic active ingredientsDigestive systemRacecadotrilAdditive ingredient
The invention provides a dry suspension agent containing racecadotril and the preparation method. The dry suspension agent provided in the invention contains a basic remedy ingredient with physiological available quantity and a carrier acceptable in pharmacy. The preparation method of the dry suspension agent containing racecadotril in the invention is characterized in that the basic remedy ingredient is firstly fixed evenly with a thinner and a suspending agent, then fixed with a wetting agent to produce granules in wet process, and finally fixed evenly with a corrective. The preparation method can hide the bitterness of medicine and guarantee the settling volume ratio of the dry suspension agent.
Owner:HAINAN SHENGKE LIFE SCI RES INST
Racecadotril lipid compostions
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Racecadotril granules and preparation technology thereof
InactiveCN104224724AImprove granulation effectMask bitternessOrganic active ingredientsDigestive systemRacecadotrilSucrose
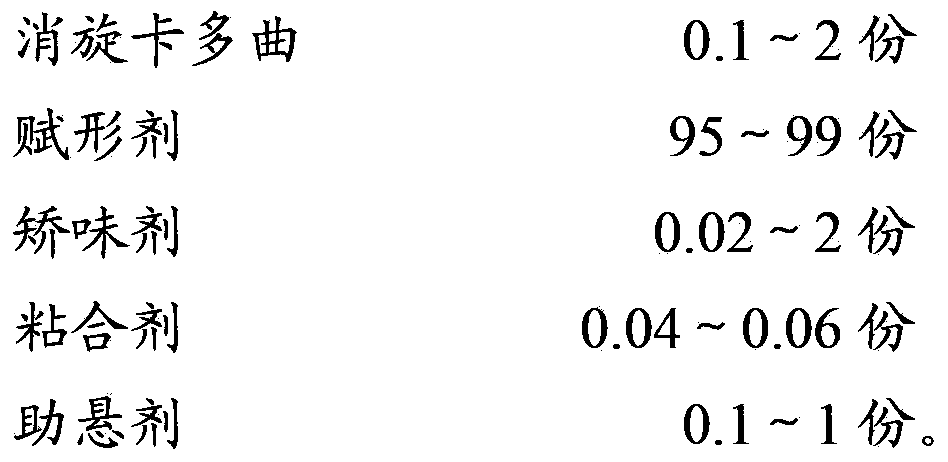
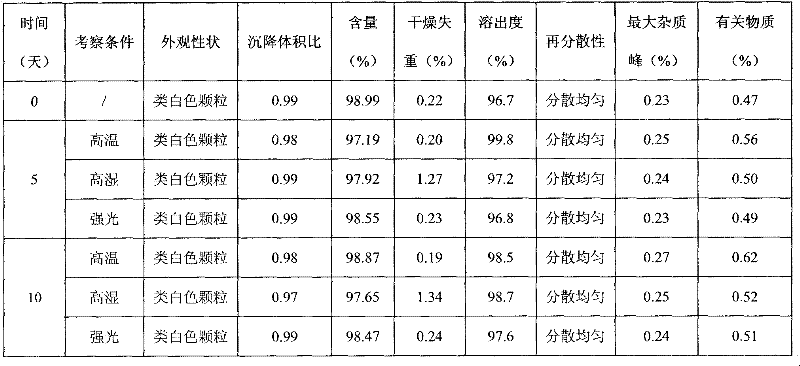
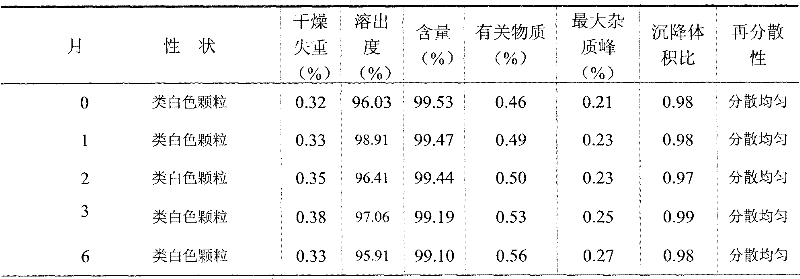
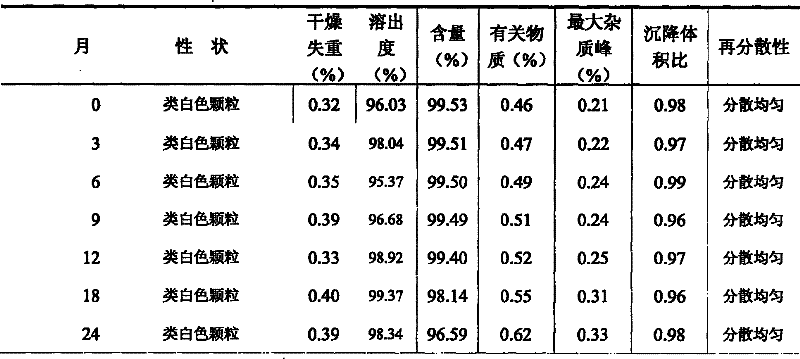
The invention relates to the field of medicinal preparations, and particularly relates to racecadotril granules and a preparation method thereof. The racecadotril granules are prepared from the following raw materials by weight: 0.1-2 parts of racecadotril, 93-99 parts of an excipient, 0.02-2 parts of a corrigent, 0.01-0.1 part of an adhesive and 0.05-1 part of a suspending agent. The racecadotril granules are prepared by suspending racecadotril in the adhesive and dissolving partial sucrose in the adhesive, so that bitter taste of a drug can be covered. The prepared racecadotril granules have relatively good granulation effect, and increase compliance of children.
Owner:BEIJING HANMI PHARMA CO LTD
Medicinal composition for treating diarrhea and preparation thereof
InactiveCN102018707AInhibition of hypersecretionInhibition of overgrowthOrganic active ingredientsDigestive systemEscherichia coliPharmaceutical Substances
The invention discloses a composition for treating diarrhea and a preparation thereof, relating to a medicinal composition for stopping the diarrhea. The invention also relates to a preparation of the medicinal composition for stopping the diarrhea. The medicinal composition is mainly prepared from the main raw materials of 1 part of Racecadotril and 0.25-25 parts of berberine hydrochloride as well as 0.01-250 parts of auxiliary materials in proportion. The medicinal composition has scientific and reasonable prescription, and can be used for inhibiting the rise of calcium ion concentration in colon smooth muscle cells and improving the motion functional coordination of the intestinal tract to realize the effect on stopping the diarrhea mainly by inhibiting the activity of enkephalinase and further inhibiting the excessive secretion of water and electrolyte via the Racecadotril, and simultaneously by the stronger bactericidal effect of the berberine hydrochloride on escherichia coli, Streptococcus hemolyticus, Diplococcus pneumoniae and the like.
Owner:KUNMING BANGYU PHARMA
Racecadotril suspension
ActiveCN102327234AImprove stabilityQuick effectOrganic active ingredientsDigestive systemRacecadotrilSucrose
The invention provides a racecadotril suspension, which is composed by the following ingredients: 1 part of racecadotril, 1-3 part(s) of mannitol, 5-9 parts of carboxymethylcellulose odium, 150-200 parts of cane sugar, 1-6 part(s) of ploxamer and 1-6 part(s) of aerosil. The racecadotril suspension has well stability and other pharmaceutical effects, because the flavoring agent is utilized, the racecadotril suspension not only solves the bad taste problem, but also surprisingly has the quick effect and high bioavailability, is better applied in clinic, and is particularly used as pediatrics pharmaceutical preparations.
Owner:HAINAN HONZ PHARMA
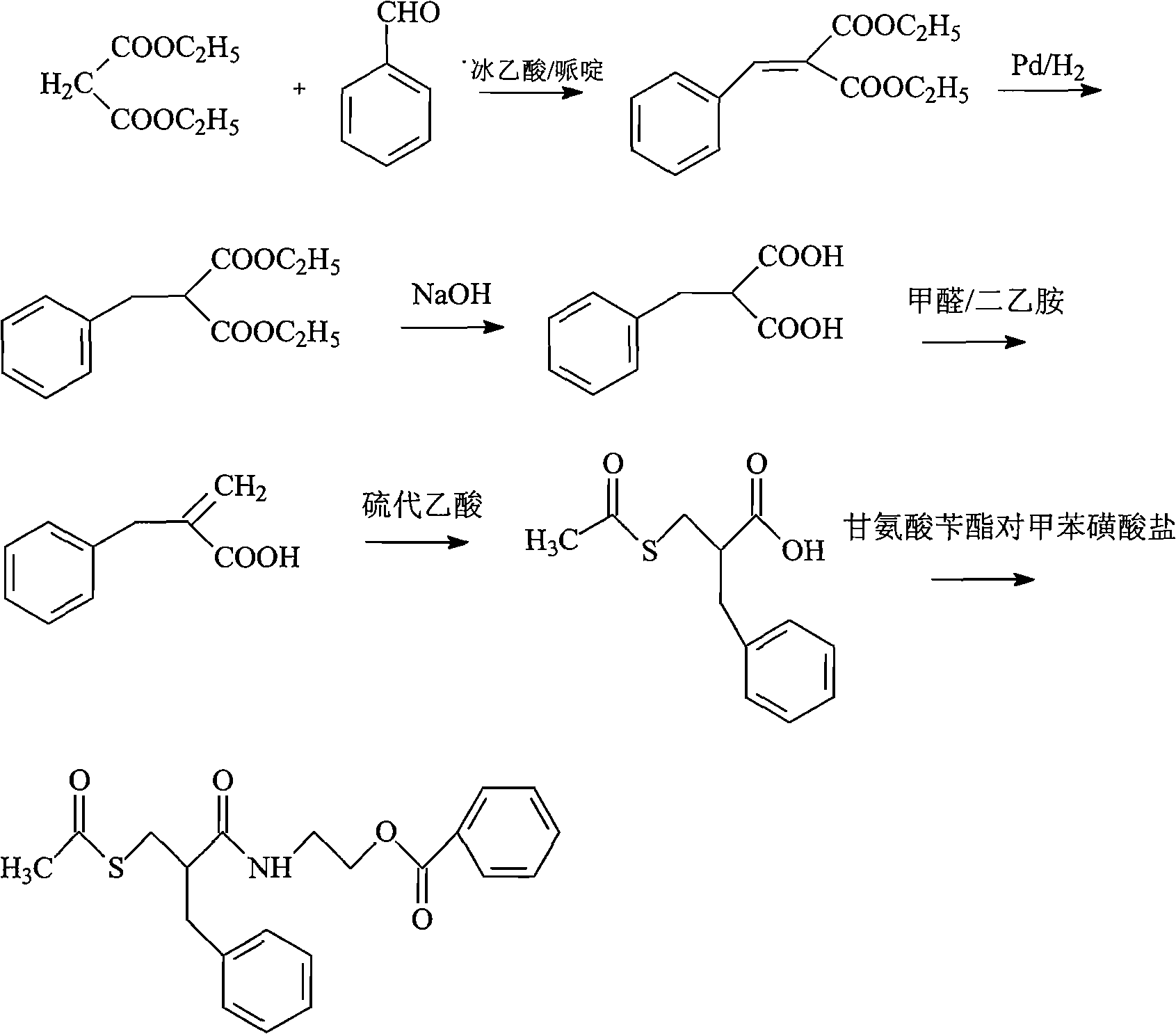
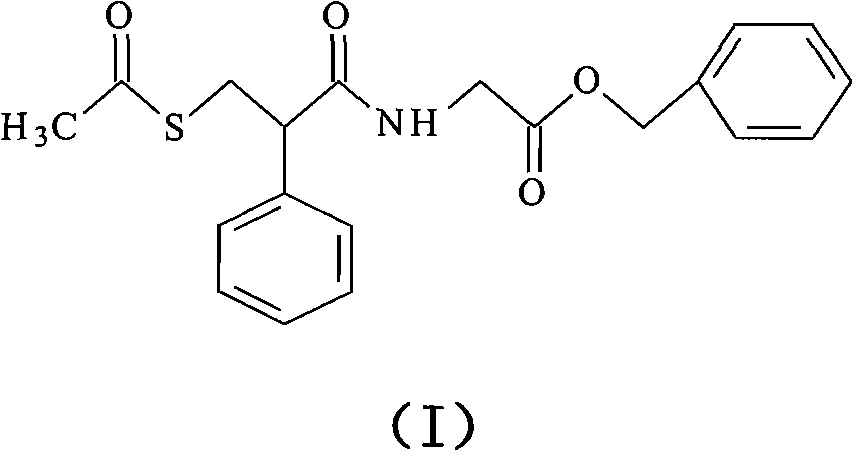
Preparation method of racecadotril
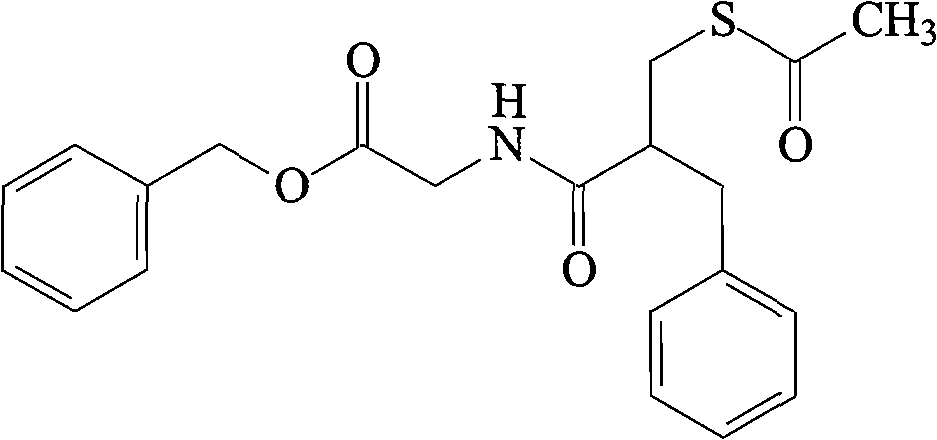
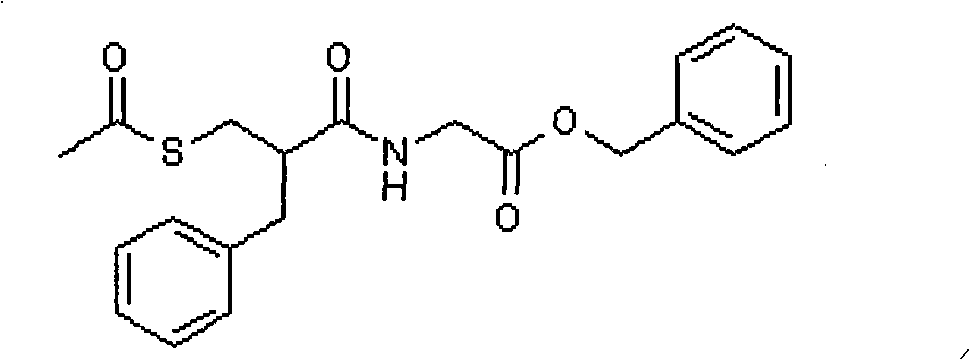
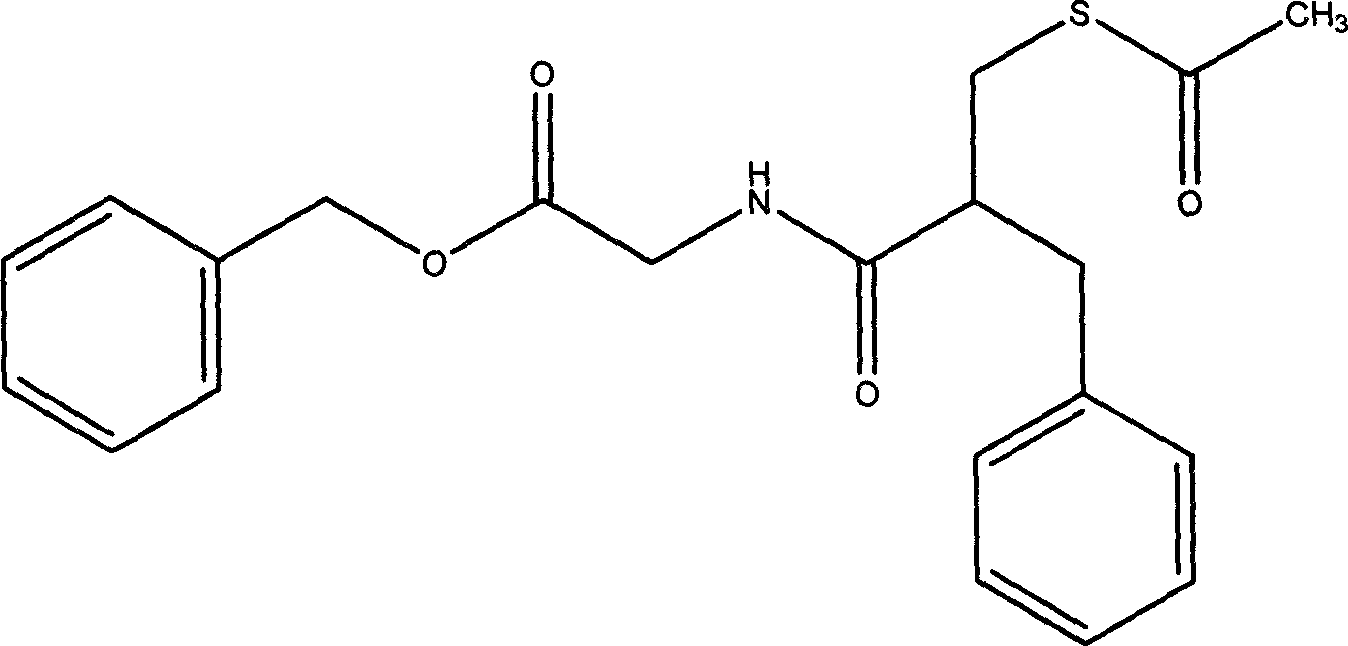
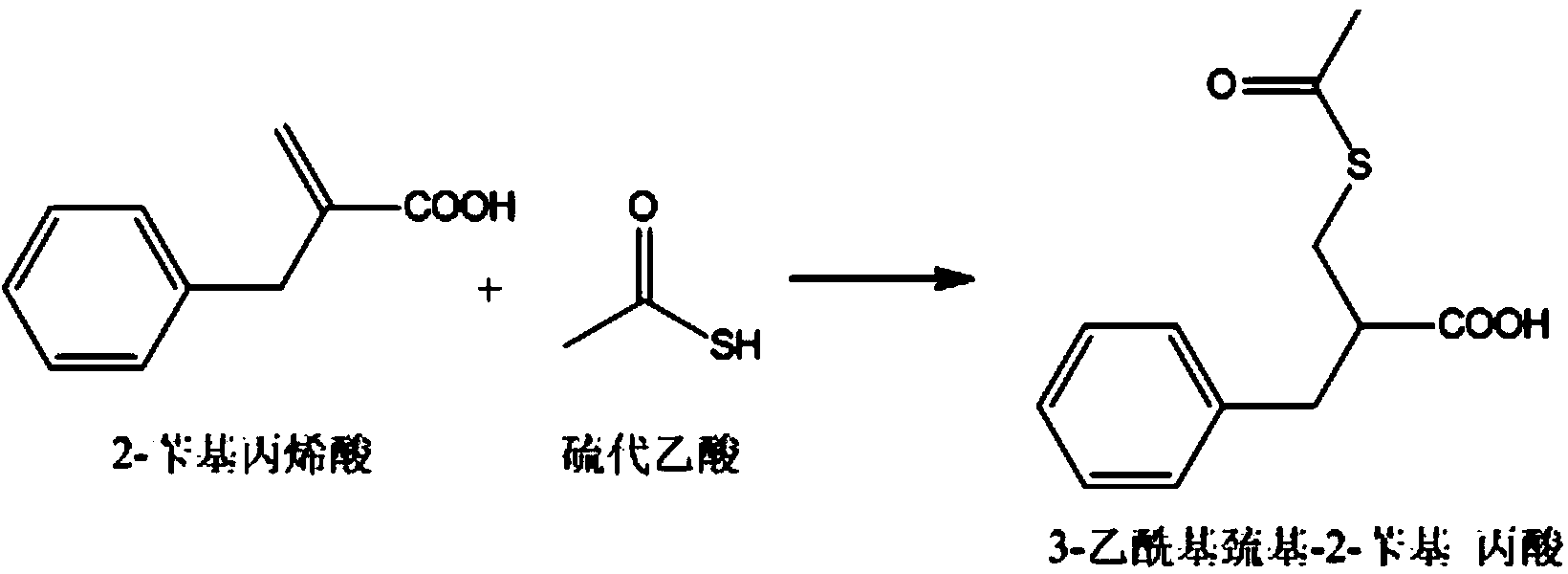
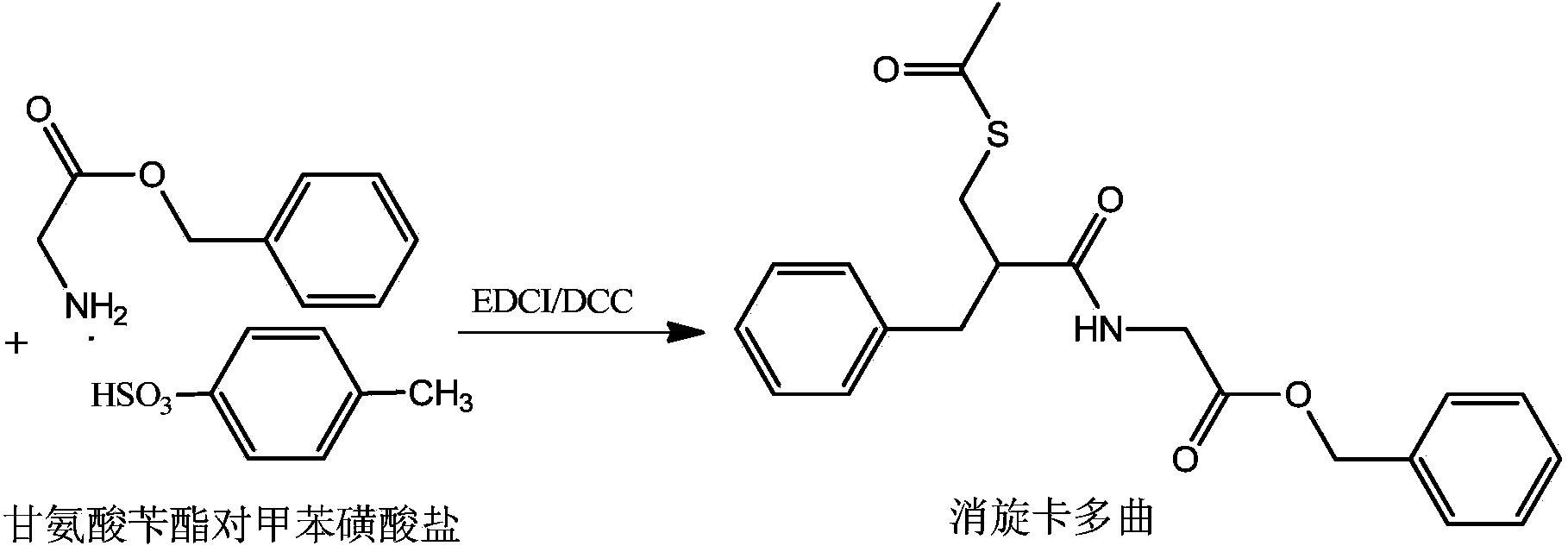
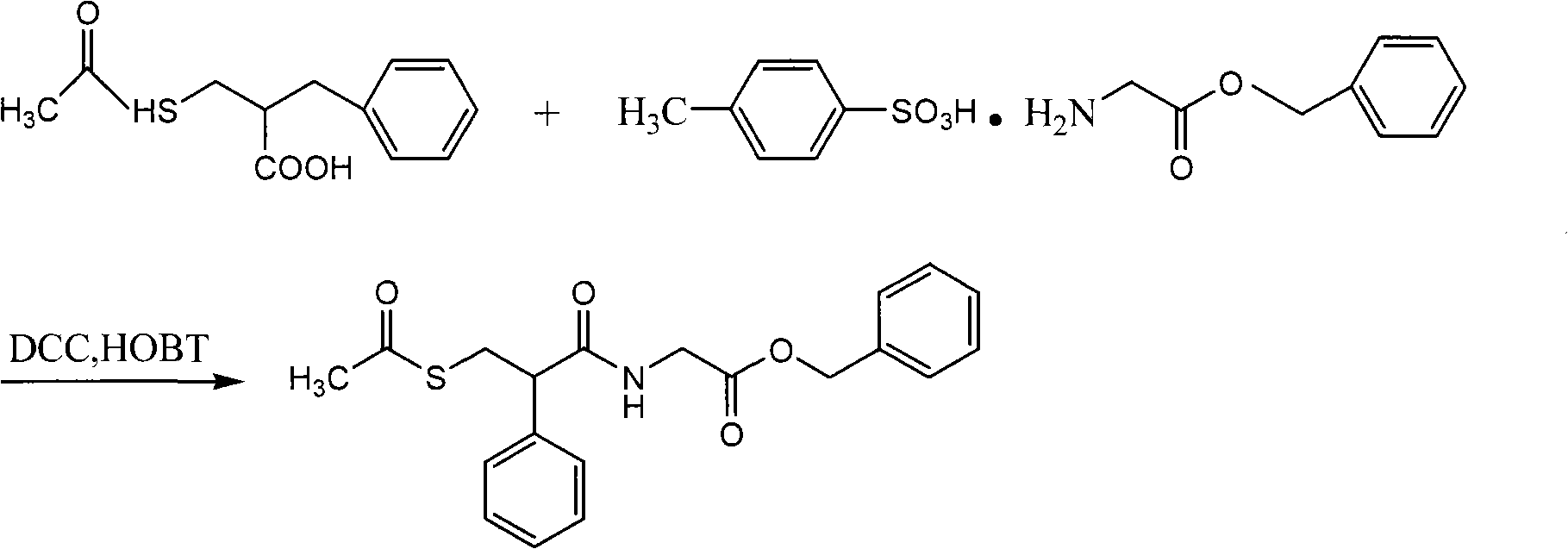
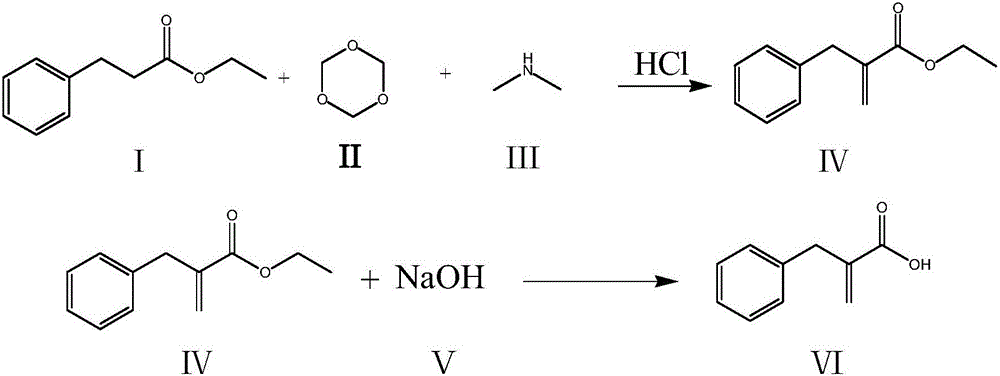
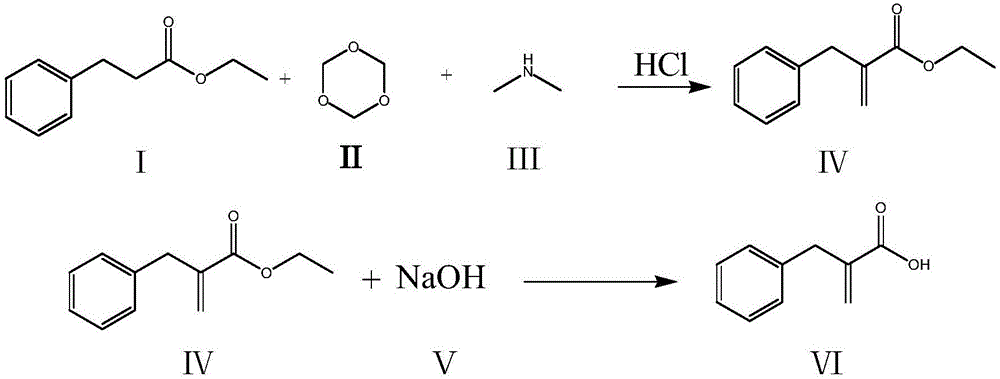
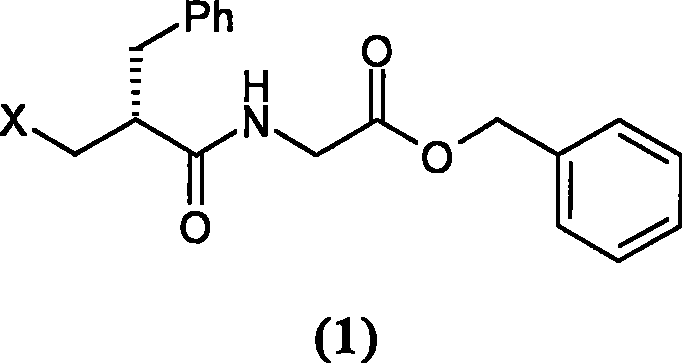
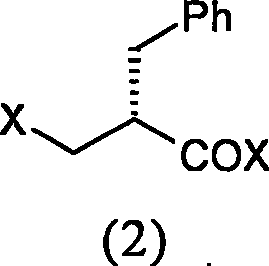
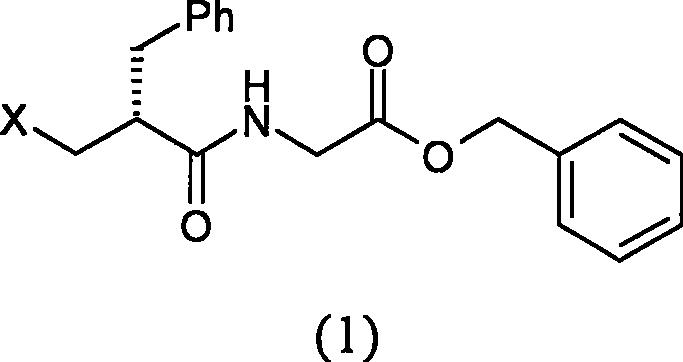
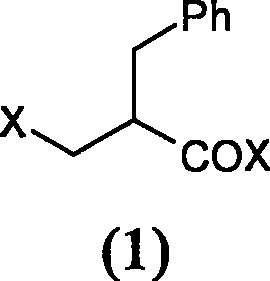
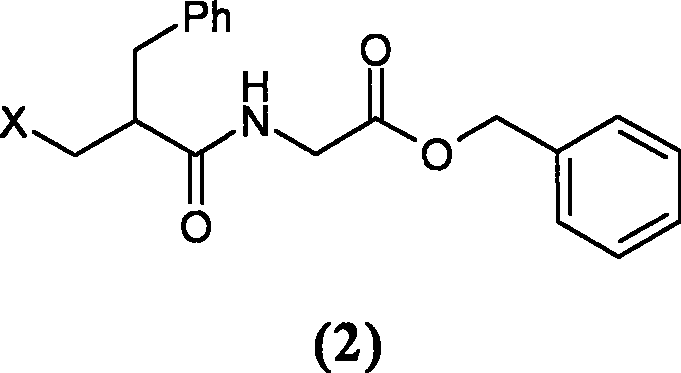
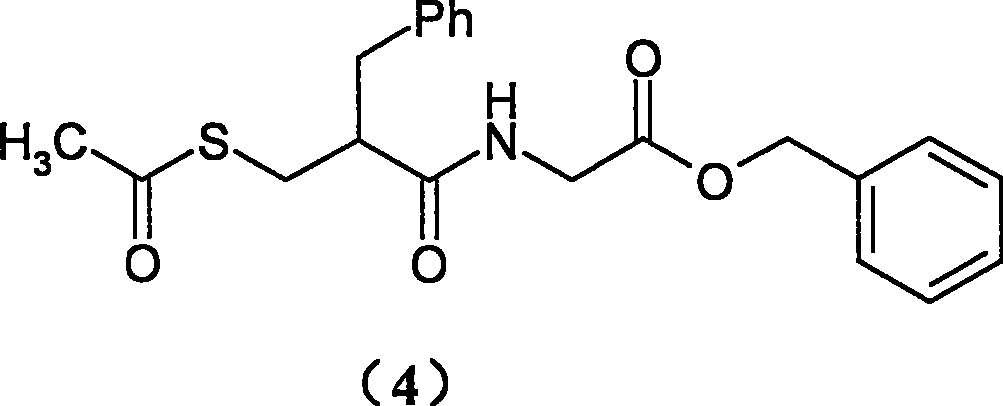
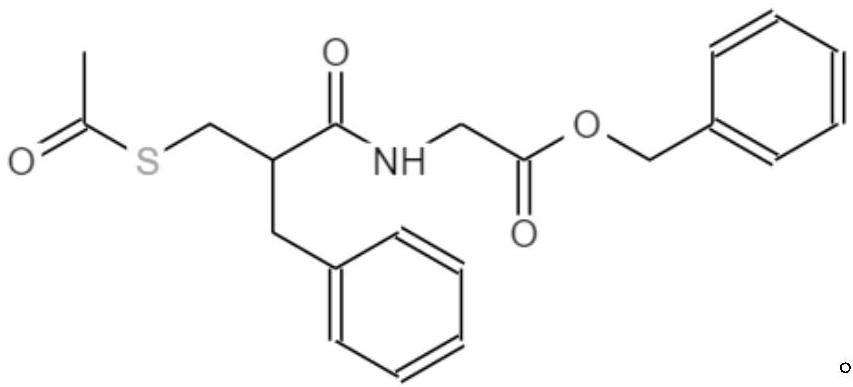
The invention relates to a preparation method of racecadotril. The method comprises the following steps: (1) enabling thioacetic acid to react with 2-benzylacrylic acid to prepare an intermediate 3-acetyl mercapto-2-benzyl propanoic acid; (2) carrying out amidation on the intermediate 3-acetyl thiol-2-glycin benzyl propionic acid and glycine benzyl ester p-toluenesulfonate salt under existence of a catalyst to generate the racecadotril. By adopting the method, the racecadotril with high yield and purity can be obtained, the defect of the original technology is compensated, pollution is reduced, and the cost is reduced. Thioacetic acid is used as a solvent and is also used as a reaction reagent, reaction process is simple and rapid, few reagents are used, and the purity and the yield are greater than or equal to 95%; EDCI or DCC is selected as a condensation reagent; a mixed solvent is used for crystallization, the influence on product yield is small, and impurity removal effect is good; the overall technology adopts a one-step to synthetize, simple, efficient, high in atom economy, more green and environment-friendly, and the racecadotril product which is high in yield, and accords with the EP quality standard can be obtained.
Owner:陕西汉江药业集团股份有限公司
Preparation method of racecadotril
InactiveCN101768095AShort reaction processHigh yieldOrganic chemistryDigestive systemAcute diarrheaGlycine
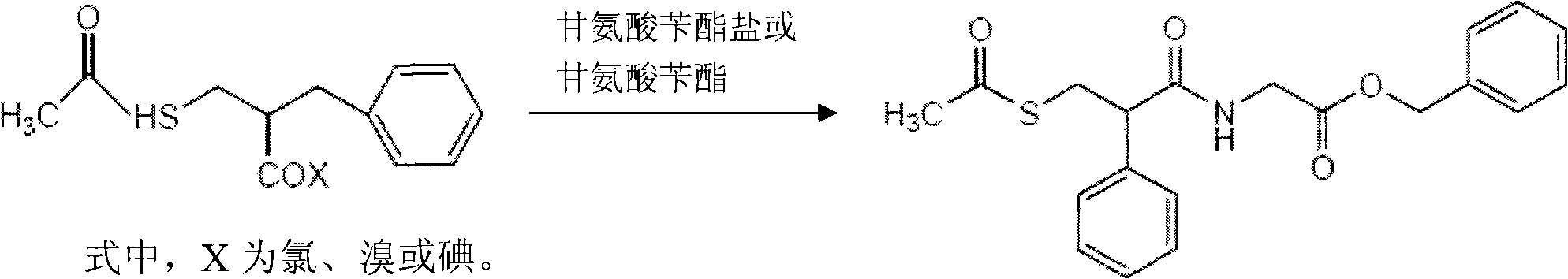
The invention relates to a preparation method of racemic N-[2-acetyl mercapto) methyl]-1-oxygen-3-phenyl propyl] glycine benzyl ester (racecadotril), which is essentially prepared by amidation reaction of 2-benzyl-3-thio-acetyl propionyl halide and glycine benzyl ester salt or glycine benzyl ester. Compared with the prior art, the invention has short reaction process, high yield. The prepared racecadotril is mainly used for the treatment of acute diarrhea for adults and children.
Owner:SHANDONG QIDU PHARMA
Racecadotril lipid compositions
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
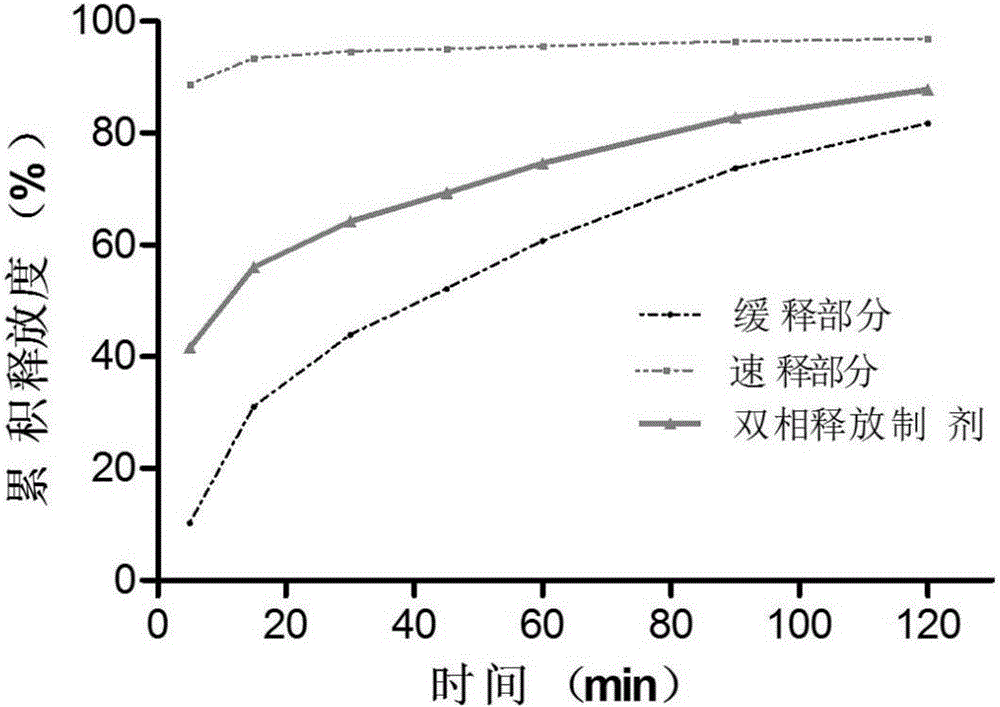
Racecadotril double-phase releasing preparation and preparation method thereof
ActiveCN106822907AIncrease contactHigh dissolution rateOrganic active ingredientsDigestive systemRacecadotrilDouble phase
The invention relates to the field of medicines, in particular to a racecadotril double-phase releasing preparation and a preparation method thereof. The racecadotril double-phase releasing preparation is prepared from medicine-containing particles with different slow-release properties and the like in percentage by mass: 10 to 90% of slow-release medicine-containing particles, 10 to 90% of quick-release medicine-containing particles, 0.1 to 0.5% of lubricant, and 0.1 to 1.0% of flavoring agent. The racecadotril double-phase releasing preparation has the advantages that the bitter taste of the racecadotril is effectively covered, the effective plasma concentration is guaranteed, the preparation technology is simple, the reproducibility is good, and the racecadotril double-phase releasing preparation is suitable for industrialized production.
Owner:SHANDONG DYNE MARINE BIOTECHCAL PHARM HLDG CO LTD
Racecadotril granules and preparation method thereof
InactiveCN110840848AImprove solubilityIncrease dissolution rateOrganic active ingredientsDigestive systemRacecadotrilPolyethylene glycol
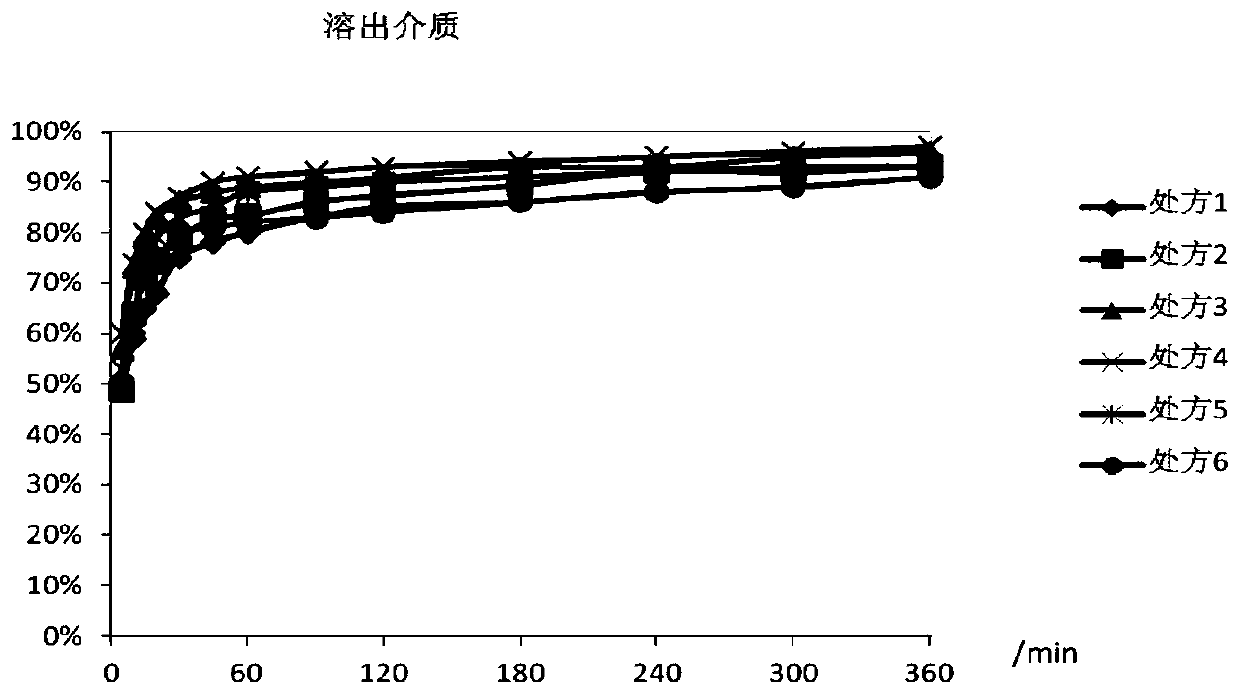
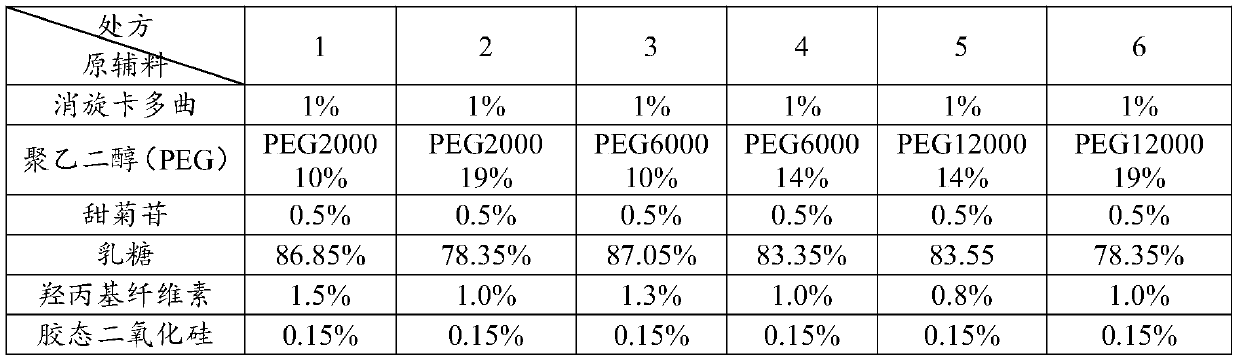
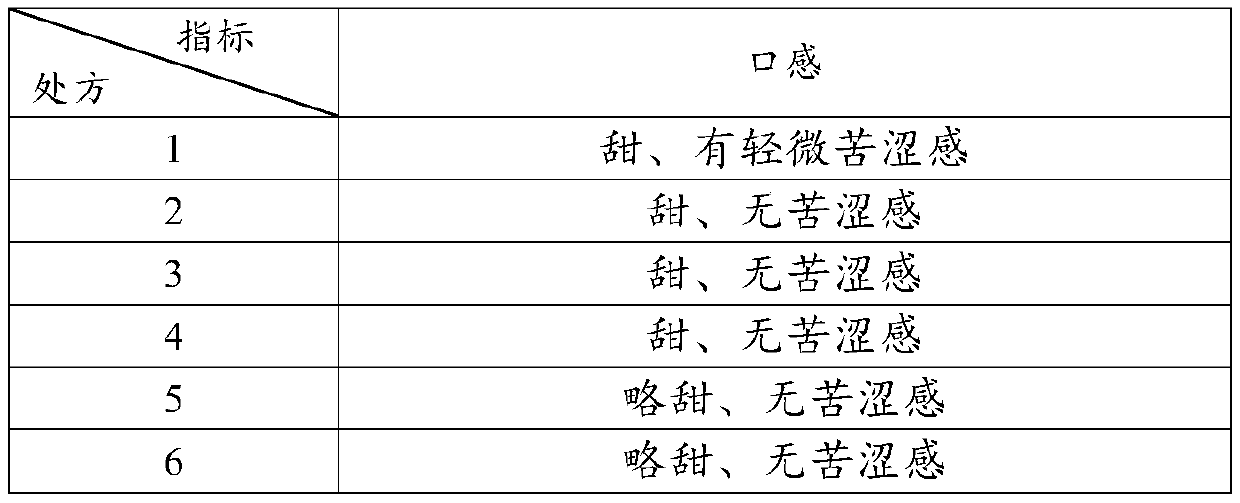
The invention discloses racecadotril granules and a preparation method thereof. The racecadotril granules comprise, by mass, 10-20% of racecadotril solid dispersions, 80-90% of fillers, 0.1-1% of flavoring agent, 0.5-1.5% of binders and 0.05-0.15% of glidant. The racecadotril solid dispersions comprise, by mass, 5-20% of racecadotril and 80-95% of polyethylene glycol. The racecadotril granules andthe technological preparation method thereof have the advantages that the bitter taste of the medicine can be covered up, the taste of the medicine can be improved, the compliance of children duringmedication can be enhanced, and the dissolution rate and stability of products can be further increased.
Owner:JIANMIN PHARMA GRP CO LTD
Racecadotril liquid compositions
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Racecadotril particle as well as preparation method thereof
InactiveCN103565750AGood water solubilityImprove bad tasteOrganic active ingredientsDigestive systemInfantile diarrheaRacecadotril
The invention belongs to the field of pharmaceutical preparations, and in particular relates to a racecadotril particle for treating infantile diarrhea as well as a preparation method thereof. The pharmaceutical particle is a solid preparation which is prepared in the following steps: firstly, carrying out clathration on racecadotril by cyclodextrin or a cyclodextrin derivative; and then, matching with a diluent, an adhesive, a disintegrating agent, a flow aid and a corrigent or other pharmaceutically accepted auxiliary materials. The particle provided by the invention effectively solves the problem of difficult administration and bad taste for children and better satisfies the clinical demands.
Owner:BEIJING D VENTUREPHARM TECH DEV
Dry mixed suspension containing racecadotril and preparation method thereof
ActiveCN101103960BGreat tasteSolving the Settling Volume Ratio ProblemOrganic active ingredientsDigestive systemRacecadotrilAdditive ingredient
The invention provides a dry suspension agent containing racecadotril and the preparation method. The dry suspension agent provided in the invention contains a basic remedy ingredient with physiological available quantity and a carrier acceptable in pharmacy. The preparation method of the dry suspension agent containing racecadotril in the invention is characterized in that the basic remedy ingredient is firstly fixed evenly with a thinner and a suspending agent, then fixed with a wetting agent to produce granules in wet process, and finally fixed evenly with a corrective. The preparation method can hide the bitterness of medicine and guarantee the settling volume ratio of the dry suspension agent.
Owner:HAINAN SHENGKE LIFE SCI RES INST
Racecadotril compound and novel preparation method thereof
InactiveCN102093272AHigh purityHigh purity and high yieldOrganic active ingredientsOrganic chemistryAcetic acidSide effect
The invention relates to a racecadotril compound and a novel preparation method thereof. In particular, neutral alumina with a specific size is taken as a fixed phase, a mixed solvent consisting of ethanol and ethyl acetate according to a certain proportion is taken as a mobile phase, and a column temperature is kept higher than the room temperature, so that racecadotril can be refined and purified efficiently, high yield and high purity are achieved, and the method is an effective method for obtaining high-purity racecadotril. By the racecadotril obtained by the method, the product quality of a preparation is enhanced, and the clinical toxic and side effects of the racecadotril compound on the preparation of antidiarrheic medicaments are reduced.
Owner:HAINAN MEIDA PHARMA
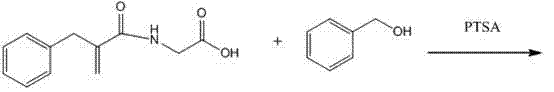
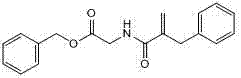
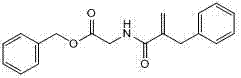
Preparation method of racecadotril intermediate 2-(benzyl acrylamide) benzyl acetate
ActiveCN102391146ALow priceReduce manufacturing costOrganic compound preparationCarboxylic acid amides preparationManufacturing cost reductionAcetic acid ear
The invention discloses a preparation method of racecadotril intermediate 2-(benzyl acrylamide) benzyl acetate, which comprises the following steps of: (1) taking 2-benzyl acrylic acid halide to react with glycinate so as to prepare 2-(benzyl acrylamide) acetic acid for standby; and (2) taking the 2-(benzyl acrylamide) acetic acid to be subjected to the catalytic esterification reaction with benzyl alcohol so as to prepare the 2-(benzyl acrylamide) benzyl acetate. In the step (1), the reaction between the 2-benzyl acrylic acid halide and the glycinate is carried out in a mixed phase of an organic solvent and an aqueous phase, and the reaction between the 2-benzyl acrylic acid halide and the glycinate is carried out in the presence of an acid-binding agent; in the step (2), the molar ratio of the 2-(benzyl acrylamide) acetic acid to the benzyl alcohol is 1:1.5-2, the catalyst is sodium toluenesulfonate or N,N-dimethylamino pyridine, and the use amount of the catalyst is 1%-2% of the molar weight of the 2-(benzyl acrylamide) acetic acid. With the adoption of the preparation method of the racecadotril intermediate 2-(benzyl acrylamide) benzyl acetate, the defects of the prior art can be overcome, and thus, the pollution is lowered, and the manufacture cost is lowered.
Owner:SHANDONG BOYUAN PHARM CO LTD
Racecadotril liposome solid preparation
InactiveCN102133186BImprove solubilityDistribute quicklyOrganic active ingredientsDigestive systemSolubilitySide effect
The invention discloses a racecadotril liposome solid preparation. The racecadotril liposome is prepared from racecadotril, soy lecithin, hydrogenated egg lecithin, cholesterol and tween 80 in the weight ratio of 1:2-8: 2-8:1.5-6:0.5-3; and the solid preparation is prepared from the racecadotril liposome and other pharmaceutically common auxiliary materials. The other pharmaceutically common auxiliary materials comprise filler, a disintegrating agent, a sweetener, an adhesive and a lubricant. The racecadotril liposome solid preparation improves the dissolubility of the racecadotril so as to improve bioavailability; a medicament is absorbed and distributed in a body fast; a curative effect is improved obviously; and the product quality of the preparation is improved and the toxic and side effects are reduced.
Owner:HAINAN MEIDA PHARMA
Compound oral liquid for treating children diarrhea and preparation method thereof
InactiveCN105920606AImprove the environmentPromote digestion and absorptionHeavy metal active ingredientsDispersion deliverySodium lactateSide effect
The invention discloses a compound oral liquid for treating children diarrhea and a preparation method thereof. The compound oral liquid for treating the children diarrhea comprises the following components of Nutrilite Double powder, dideoxyinosine, gastrodia elata polypeptide, disaccharidase, ribavirin, racecadotril, bismuth pectin, hydrotalcite, dry yeast, probiotics, potassium chloride, sodium chloride, sodium lactate, calcium gluconate, corrigent, emulsifying agent, buffer liquid, and antioxidant. The compound oral liquid for treating the children diarrhea has the advantages that the environment of children gastrointestinal tracts is improved, the effective microbial community is increased, the digestion and adsorbing of gastrointestinal tract are promoted, the water-electrolyte metabolic disorder is prevented, and the body immunity is improved; the cost is low, the toxic or side effect is little, the compound oral liquid can be used for a long time, and the children diarrhea can be prevented and thoroughly cured after long-time administration.
Owner:钟志敏
Racecadotril lipid compositions
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
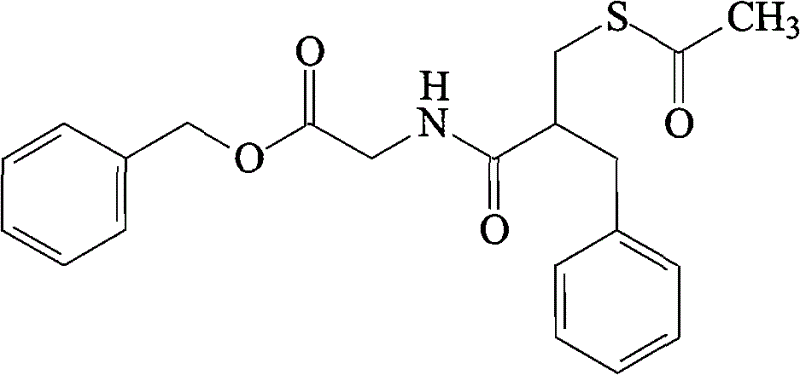
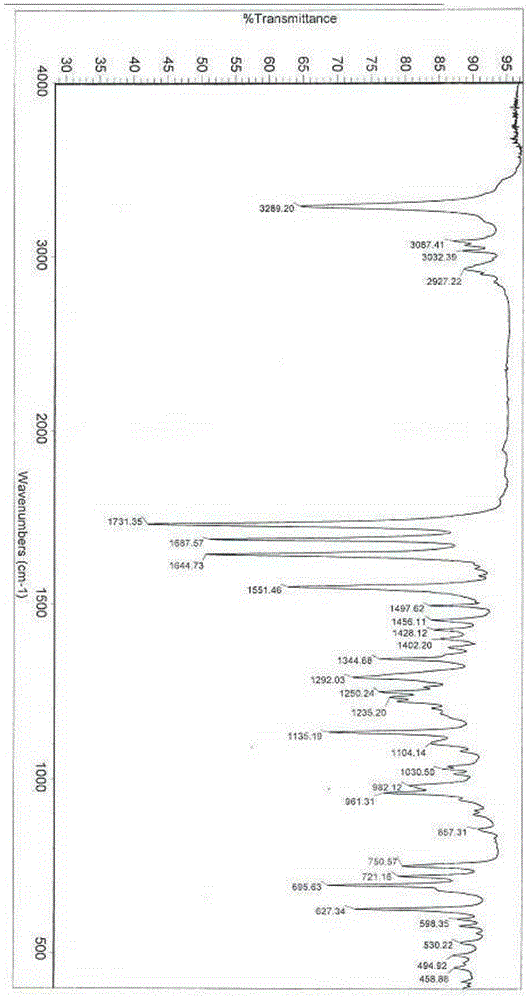
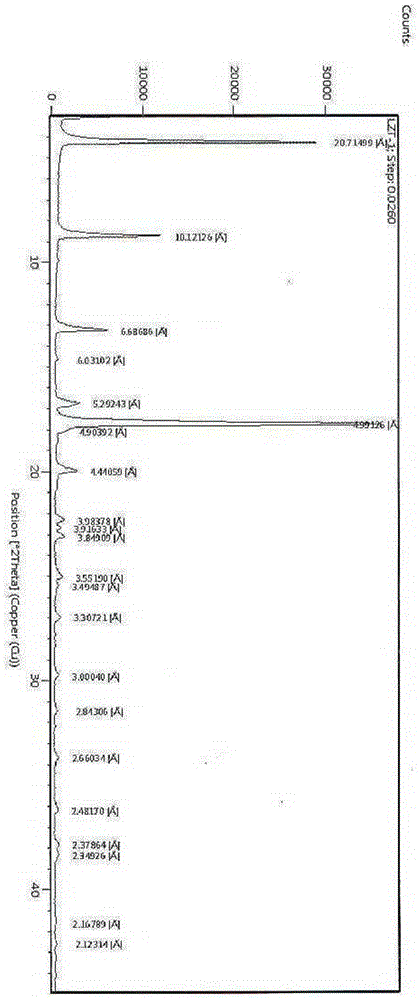
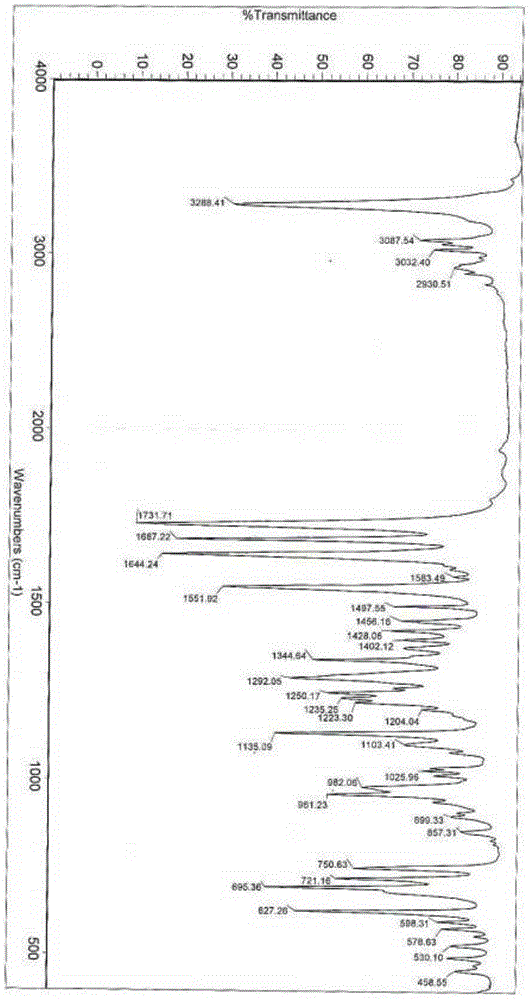
Alpha crystal form of racecadotril and preparation method of alpha crystal form
InactiveCN104356036ACrystal stableHigh purityOrganic active ingredientsOrganic chemistryRacecadotrilX-ray
The invention belongs to the technical field of crystal forms and preparation of drugs and particularly relates to an alpha crystal form of racecadotril and a preparation method of the alpha crystal form. The alpha crystal form of racecadotril is characterized in that the reflection angle 2theta shows X-ray powder diffraction peaks at sites of 4.2-4.3 degrees, 8.7-8.8 degrees, 13.2-13.3 degrees, 16.7-17.8 degrees, 17.7-17.8 degrees and 19.9-20.0 degrees and shows infrared absorption peaks at sites of 1135-1136cm<-1>, 1551-1553cm<-1>, 1644-1645cm<-1>, 1687-1688cm<-1>, 1731-1732cm<-1> and 3287-3290cm<-1> in an X-ray powder diffraction diagram. The preparation method comprises the following steps of dissolving racecadotril in a solvent, heating until racecadotril is dissolved, then standing still for crystallization, filtering, and drying. The prepared alpha crystal form of racecadotril is high in purity and stable; the preparation method is simple in process and easy to implement, the yield is 70%-80%, and the purify of the alpha crystal form is more than or equal to 99.0%.
Owner:SHANDONG QIDU PHARMA
Racecadotril granules and preparation method thereof
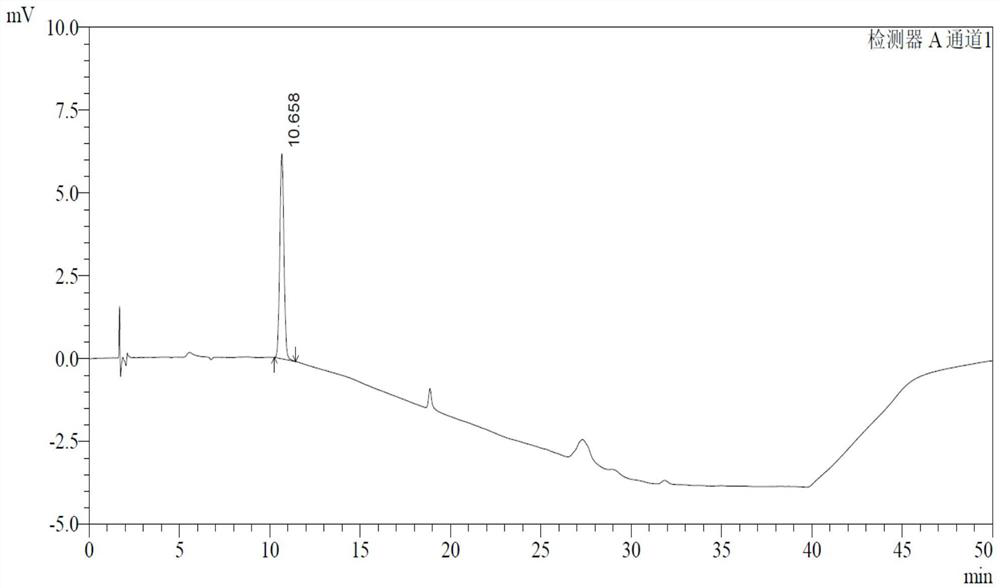
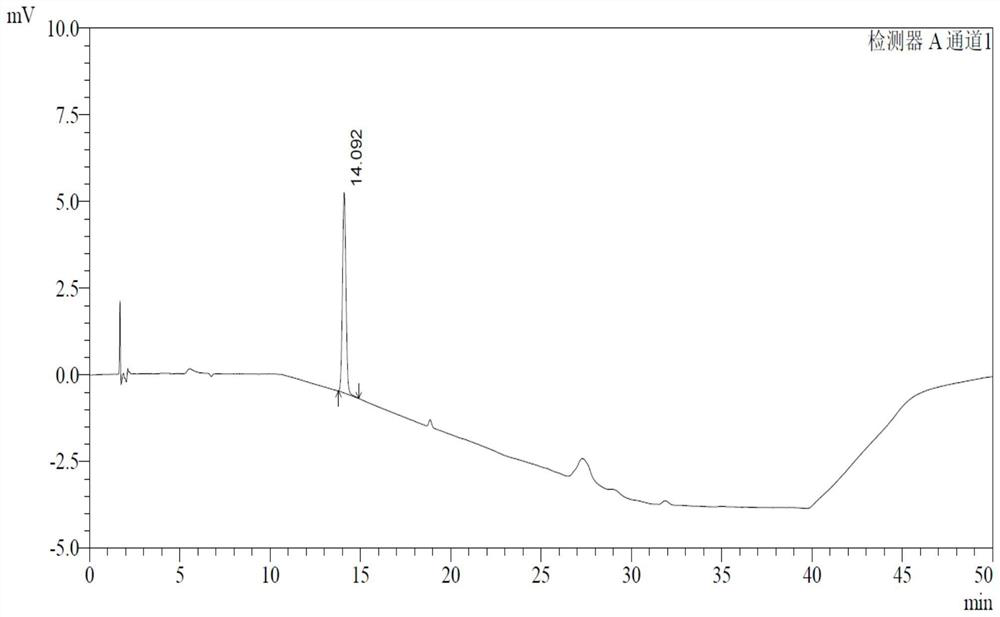
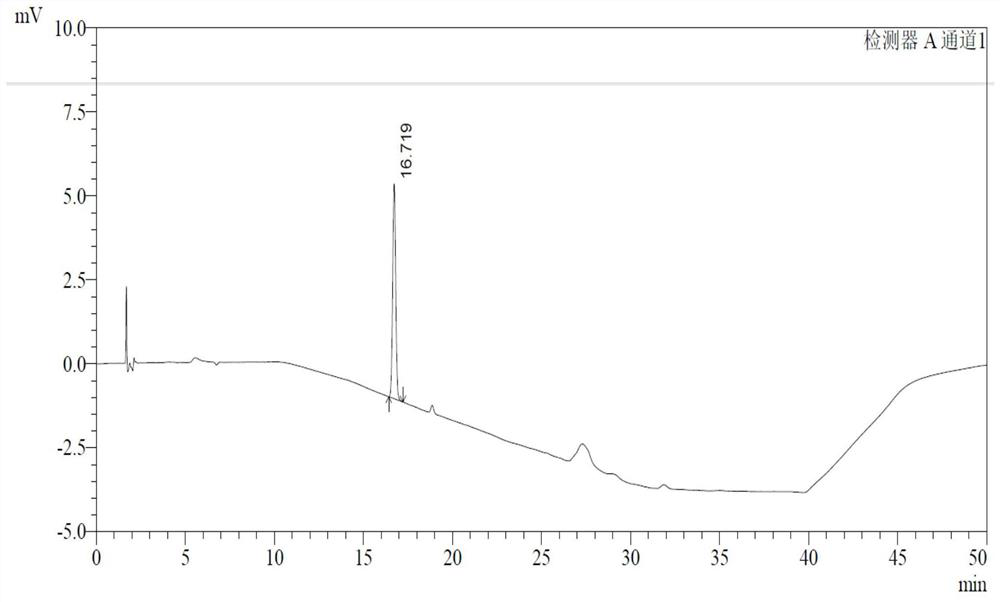
ActiveCN107811973BHigh dissolution rateWell mixedOrganic active ingredientsDigestive systemSolubilityRacecadotril
The invention discloses racecadotril granules. Based on 100 bags, the racecadotril granules comprise the following raw medicinal materials and medicinal auxiliary materials in parts by weight: 5g-15gof racecadotril, 1g-5g of coconut oil, 800g-1200g of filler, 100g-200g of an adhesive and 100g-200g of ethanol. The invention also discloses a preparation method of the racecadotril granules. The preparation method comprises the following steps: mixing and stirring ethanol and coconut oil to form a micro-emulsion to increase the solubility of racecadotril; and then adding 1-5% of filler, wherein asmall amount of filler can play a role in a cosolvent accidently, so that the dissolution rate of the racecadotril granules is improved greatly. By adding a lot of filler to modify the taste and cover the bitterness of racecadotril, so that the taste of a drug is improved. The racecadotril granules avoid phenomena that the racecadotril granules prepared by an existing preparation method are poorin taste, poor in dissolution rate and hard to store, and the percent of pass of the product is improved greatly.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
Compounding method for racecadotril intermediate
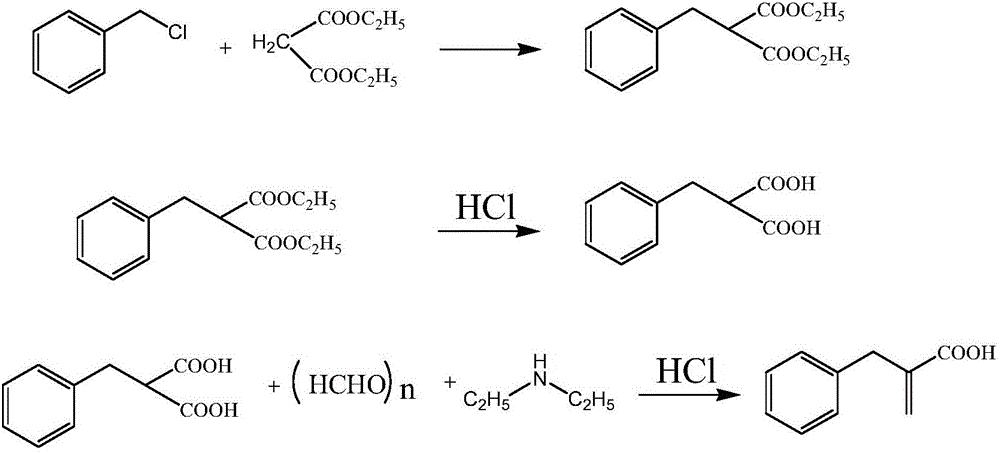
ActiveCN106542961AShort synthetic routeHigh yieldOxygen-containing compound preparationOrganic compound preparationRacecadotrilBenzyl chloride
The invention discloses a compounding method for a racecadotril intermediate. The compounding method comprises the following steps: causing ethyl phenylpropiolate, dimethylamine and trioxymethylene react with each other, treating with hydrochloric acid to obtain benzyl ethyl acrylate, and then hydrolyzing under an alkaline condition, thereby obtaining benzyl acrylic acid. According to the compounding method disclosed by the invention, benzyl chloride with higher toxicity is replaced by ethyl phenylpropiolate, the compounding route is shortened, the production efficiency is increased, and thus the compounding method is suitable for industrial production.
Owner:SHANDONG BOYUAN PHARM CO LTD
Medicinal preparations containing racecadotril and preparation methods
InactiveCN105125477AEasy to takeGood treatment effectOrganic active ingredientsAerosol deliveryDrugDiarrhea
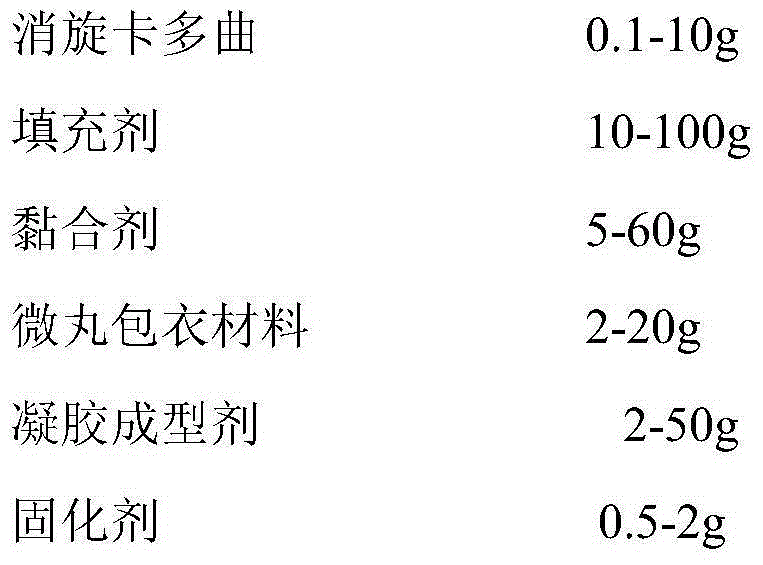
The invention discloses medicinal preparations containing racecadotril and preparation methods, wherein racecadotril oral pellet gel is prepared from the following main ingredients in parts by mass: 0.01-10 parts of racecadotril, 10-100 parts of filling agent, 5-60 parts of binding agent, 2-20 parts of pellet coating material, 2-50 parts of gel forming agent, 0.5-2 parts of curing agent, 0.2-200 parts of sweetening agent, 0.5-5 parts of flavoring agent, 0.1-1.5 parts of preservative and 0-30 parts of pH conditioning agent; racecadotril oral pellet emulsion is prepared from the following main ingredients in parts by mass: 0.01-10 parts of racecadotril, 10-100 parts of filling agent, 5-60 parts of binding agent, 2-20 parts of pellet coating material, 50-350 parts of oil phase, 1-300 parts of emulgator and 0-150 parts of co-emulsifier. The medicinal preparations disclosed by the invention have the beneficial effects that the racecadotril oral pellet products are easy to take by children, and have a good treatment effect for diarrhea of children.
Owner:黑龙江童医生儿童生物制药有限公司
Intermediate compound for preparing dextro cadotril and its preparation process and use
ActiveCN100537522CThree wastes lessFew reaction stepsOrganic compound preparationCarboxylic acid amides preparationRacecadotrilCombinatorial chemistry
Owner:SHANGHAI SHYNDEC PHARMA CO LTD +1
Racecadotril lipid compositions
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Preparation process of cadotril
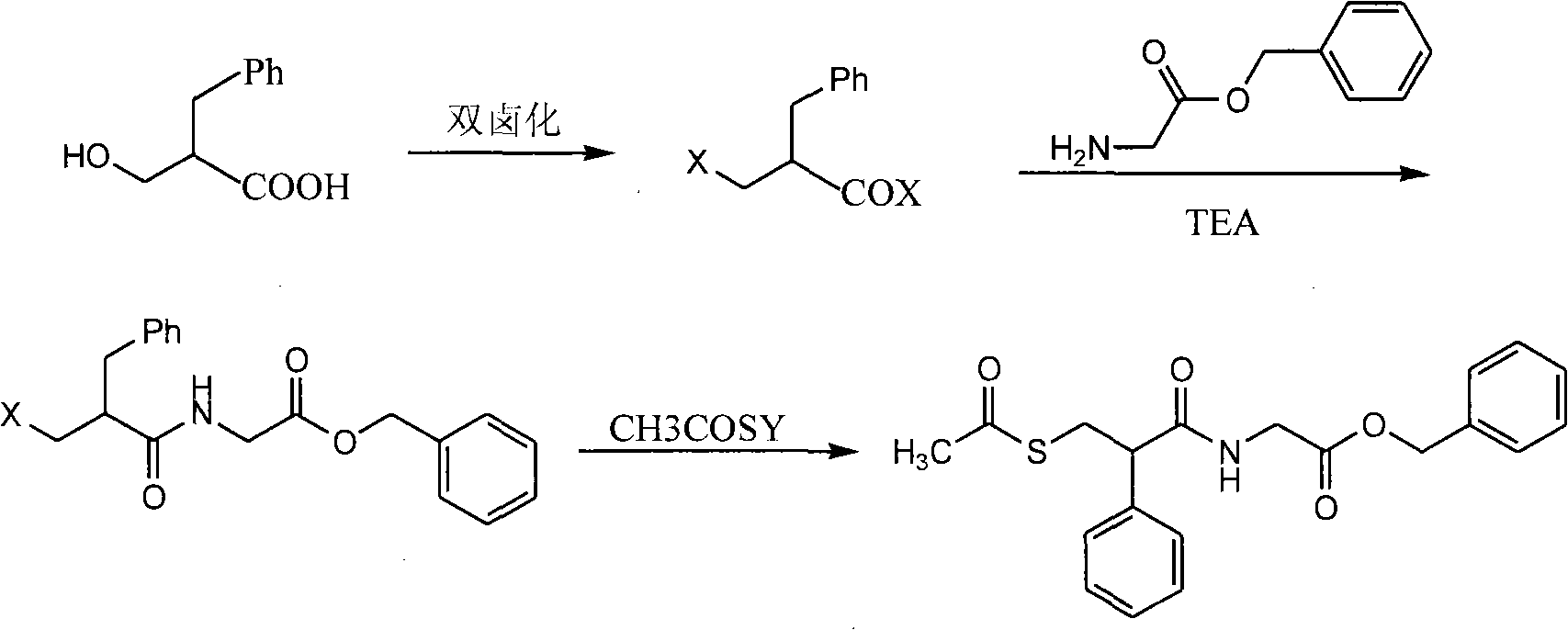
ActiveCN100537531CThree wastes lessFew reaction stepsOrganic chemistry3-Hydroxypropionic acidRacecadotril
The invention discloses a method for preparing cadotril. The method uses 2-benzyl-3-hydroxypropionic acid as a raw material to prepare cadotril through halogenation, amidation and substitution. The raw materials and reagents used in the route are all The method is cheap and easy to obtain, has less three wastes, is simple to operate, has few reaction steps and high yield, and is suitable for industrial production.
Owner:SHANGHAI SHYNDEC PHARMA CO LTD +1
Racecadotril granules and preparation method thereof
ActiveCN107811973ALow costQuality is easy to controlOrganic active ingredientsDigestive systemSolubilityEmulsion
The invention discloses racecadotril granules. Based on 100 bags, the racecadotril granules comprise the following raw medicinal materials and medicinal auxiliary materials in parts by weight: 5g-15gof racecadotril, 1g-5g of coconut oil, 800g-1200g of filler, 100g-200g of an adhesive and 100g-200g of ethanol. The invention also discloses a preparation method of the racecadotril granules. The preparation method comprises the following steps: mixing and stirring ethanol and coconut oil to form a micro-emulsion to increase the solubility of racecadotril; and then adding 1-5% of filler, wherein asmall amount of filler can play a role in a cosolvent accidently, so that the dissolution rate of the racecadotril granules is improved greatly. By adding a lot of filler to modify the taste and cover the bitterness of racecadotril, so that the taste of a drug is improved. The racecadotril granules avoid phenomena that the racecadotril granules prepared by an existing preparation method are poorin taste, poor in dissolution rate and hard to store, and the percent of pass of the product is improved greatly.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
Determination method of p-toluenesulfonate esters in racecadotril
Owner:SHANDONG BOYUAN PHARM CO LTD
Pharmaceutical preparation containing racecadotril and preparation method thereof
ActiveCN114469873AInhibit aggregationRaise the film forming temperatureOrganic active ingredientsDigestive systemDrug release rateCellulose
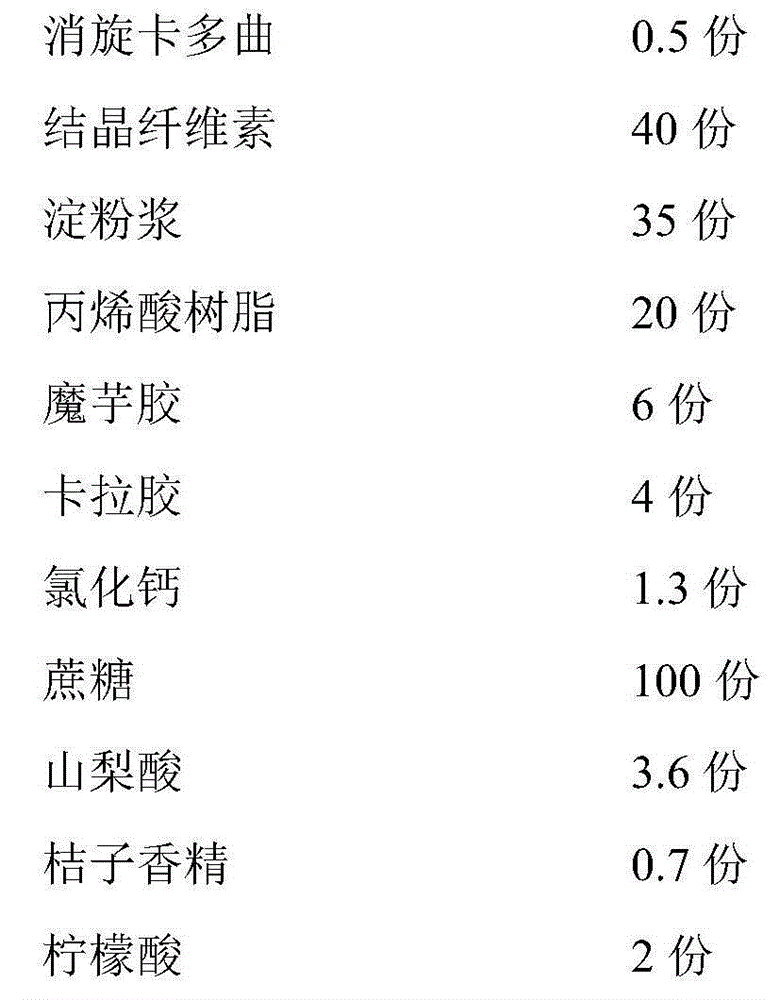
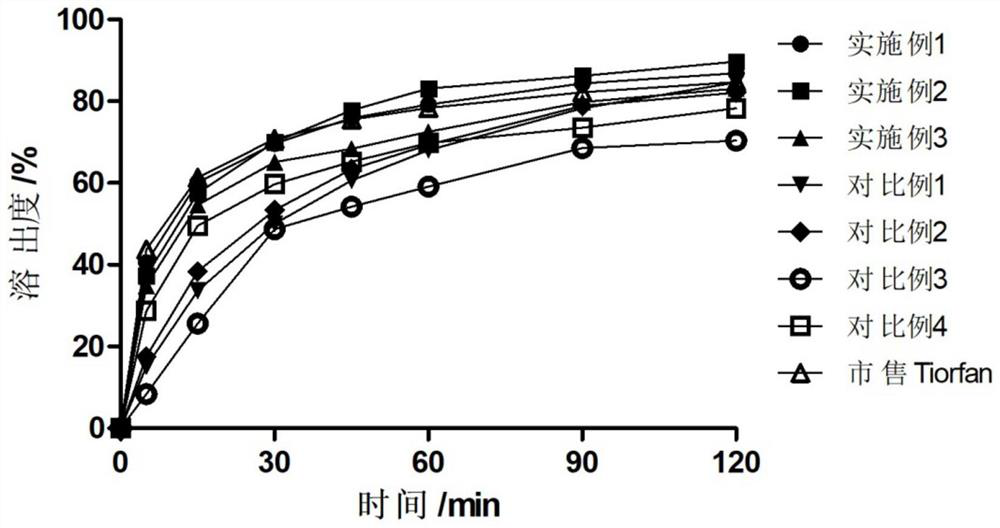
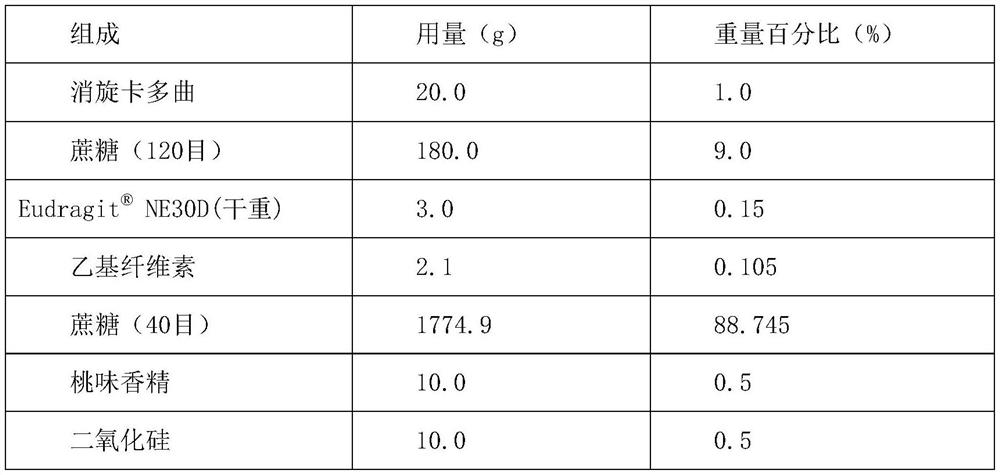
The invention discloses a pharmaceutical preparation containing racecadotril and a preparation method thereof, and belongs to the field of pharmaceutical preparations. The preparation comprises racecadotril, a taste masking material, a filling agent, a flavoring agent and a lubricant, the taste masking material is composed of NE30D and ethyl cellulose fine powder according to a certain proportion, and further aggregation of an acrylate copolymer is prevented through formation of an incompatible phase between ethyl cellulose and acrylate copolymer particles; the water vapor permeability and tension of the membrane are improved, so that the drug release rate is stabilized, the bitter taste of the drug is effectively covered, and the membrane is suitable for acute diarrhea treatment of infant and child patients; the product stability is good; the preparation method has the advantages of good drug entrapment performance, good taste masking effect, and simple preparation process by adopting a conventional wet granulation process, and is more suitable for industrial production.
Owner:BEIJING CHILDRENS HOSPITAL AFFILIATED TO CAPITAL MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com