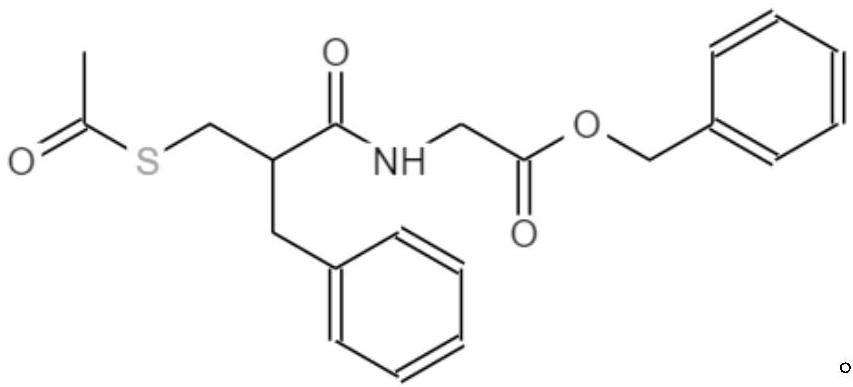
Pharmaceutical preparation containing racecadotril and preparation method thereof
A technology for racecadotril and pharmaceutical preparations, which is applied in the field of pharmaceutical preparations containing racecadotril and its preparation, and can solve the problems of insignificant taste-masking effect, reduced dissolution rate, low solubility of racecadotril, etc. , to achieve the effect of improving water vapor permeability and tension, increasing film forming temperature, and good drug loading performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
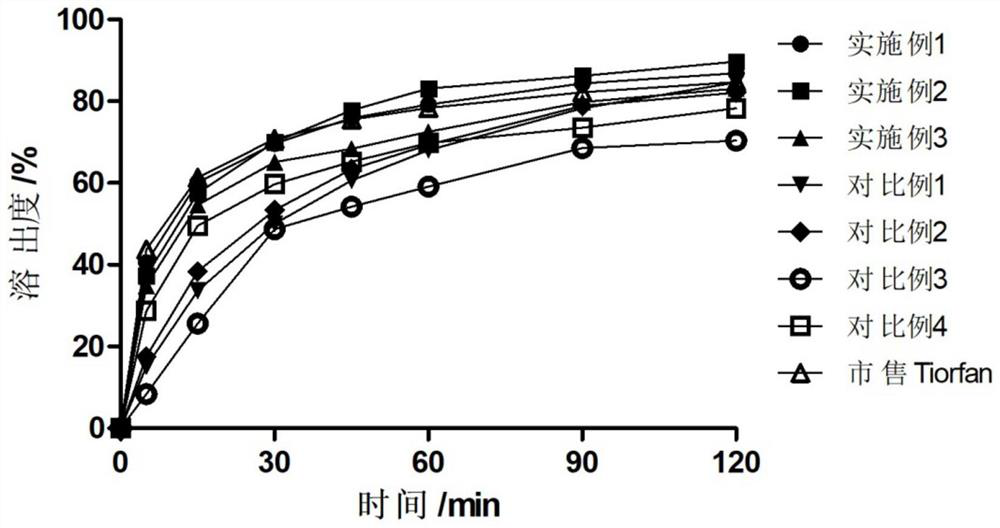
Examples
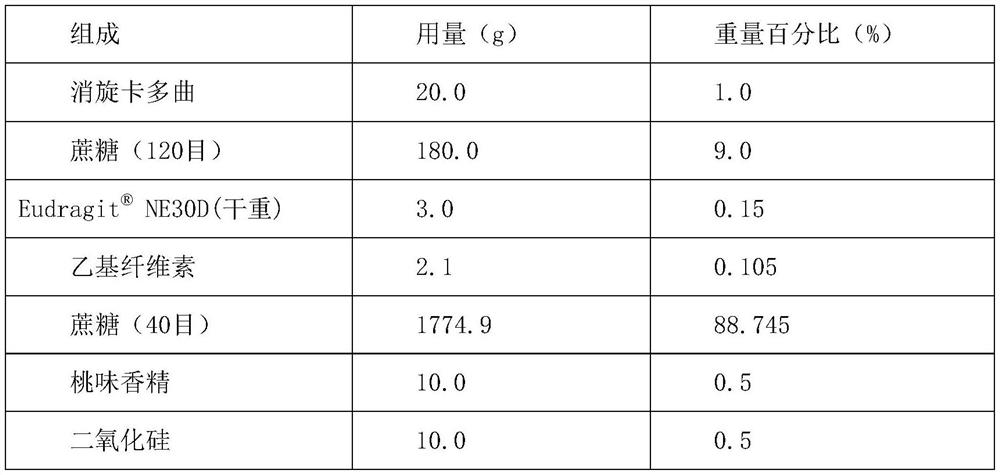
Embodiment 1
[0040] prescription:
[0041]
[0042]
[0043] Preparation Process:
[0044] (1) Pulverize the racecadotril raw material with an airflow mill to obtain racecadotril micropowder, with an average particle diameter of D 50 Control within the range of 20 μm; use a universal pulverizer to pulverize ethyl cellulose to obtain ethyl cellulose fine powder with an average particle size of 45 μm;
[0045] (2) Weighing the prescription amount NE30D, diluted with 1.5 times the amount of purified water; under the action of high shear, add ethyl cellulose fine powder to prepare a binder solution;
[0046] (3) Put racecadotril micropowder and sucrose (120 mesh) in a high-shear wet granulator, add a binder solution for wet granulation, and place the prepared wet granules in a blast drying box, Dry at 60°C for 4 hours, and obtain drug-containing granules after granulation;
[0047] (4) Mix the drug-containing granules with sucrose (40 mesh), peach essence and silicon dioxide in a th...
Embodiment 2
[0049] prescription:
[0050]
[0051] Preparation Process:
[0052] (1) Pulverize the racecadotril raw material with an airflow mill to obtain racecadotril micropowder, with an average particle diameter of D 50 Control within the range of 20 μm; use a universal pulverizer to pulverize ethyl cellulose to obtain ethyl cellulose fine powder with an average particle size of 45 μm;
[0053] (2) Weighing the prescription amount NE30D, diluted with 1.5 times the amount of purified water; under the action of high shear, add ethyl cellulose fine powder to prepare a binder solution;
[0054] (3) Put racecadotril micropowder and sucrose (120 mesh) in a high-shear wet granulator, add a binder solution for wet granulation, and place the prepared wet granules in a blast drying box, Dry at 60°C for 4 hours, and obtain drug-containing granules after granulation;
[0055] (4) Mix the drug-containing granules with sucrose (40 mesh), peach essence and silicon dioxide in a three-dimensio...
Embodiment 3
[0057] prescription:
[0058]
[0059] Preparation Process:
[0060] (1) Pulverize the racecadotril raw material with an airflow mill to obtain racecadotril micropowder, with an average particle diameter of D 50 Control within the range of 20 μm; use a universal pulverizer to pulverize ethyl cellulose to obtain ethyl cellulose fine powder with an average particle size of 20 μm;
[0061] (2) Weighing the prescription amount NE30D, diluted with 1.5 times the amount of purified water; under the action of high shear, add ethyl cellulose fine powder to prepare a binder solution;
[0062] (3) Put racecadotril micropowder and sucrose (120 mesh) in a high-shear wet granulator, add a binder solution for wet granulation, and place the prepared wet granules in a blast drying box, Dry at 60°C for 4 hours, and obtain drug-containing granules after granulation;
[0063] (4) Mix the drug-containing granules with sucrose (40 mesh), peach essence and silicon dioxide in a three-dimensio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com