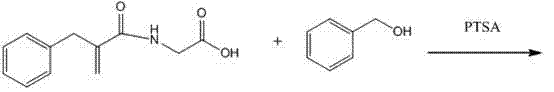
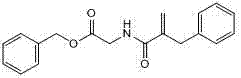
Preparation method of racecadotril intermediate 2-(benzyl acrylamide) benzyl acetate
A technology of benzyl acrylamide and racecadotril, which is applied in the field of pharmaceuticals, can solve the problems of less public reports, increased manufacturing costs, and high prices, and achieve the effects of reduced production costs, pollution avoidance, and low prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] The preparation method of the present invention comprises the following steps:
[0019] ① Take 2-benzylacrylic acid halide and glycine salt to react to make 2-(benzylacrylamide) acetic acid for later use;
[0020] ② Catalyzed esterification reaction of 2-(benzylacrylamide) acetic acid and benzyl alcohol to produce 2-(benzyl acrylamide) benzyl acetate;
[0021] The molar ratio of 2-benzyl acrylic acid halide and glycinate in step ① is 1:1-1.2; 2-benzyl acrylic acid halide and glycinate are carried out in the mixed phase of organic solvent and aqueous phase, organic The solvent is any one of methylene chloride, chloroform, toluene or acetonitrile, and the reaction of 2-benzyl acrylic acid halide and glycinate is carried out in the presence of an acid-binding agent;
[0022] The molar ratio of 2-(benzylacrylamide) acetic acid to benzyl alcohol in step ② is 1:1.5-2, the catalyst is sodium toluenesulfonate or N,N-dimethylaminopyridine, and the dosage is 2-(benzyl Acrylamid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com