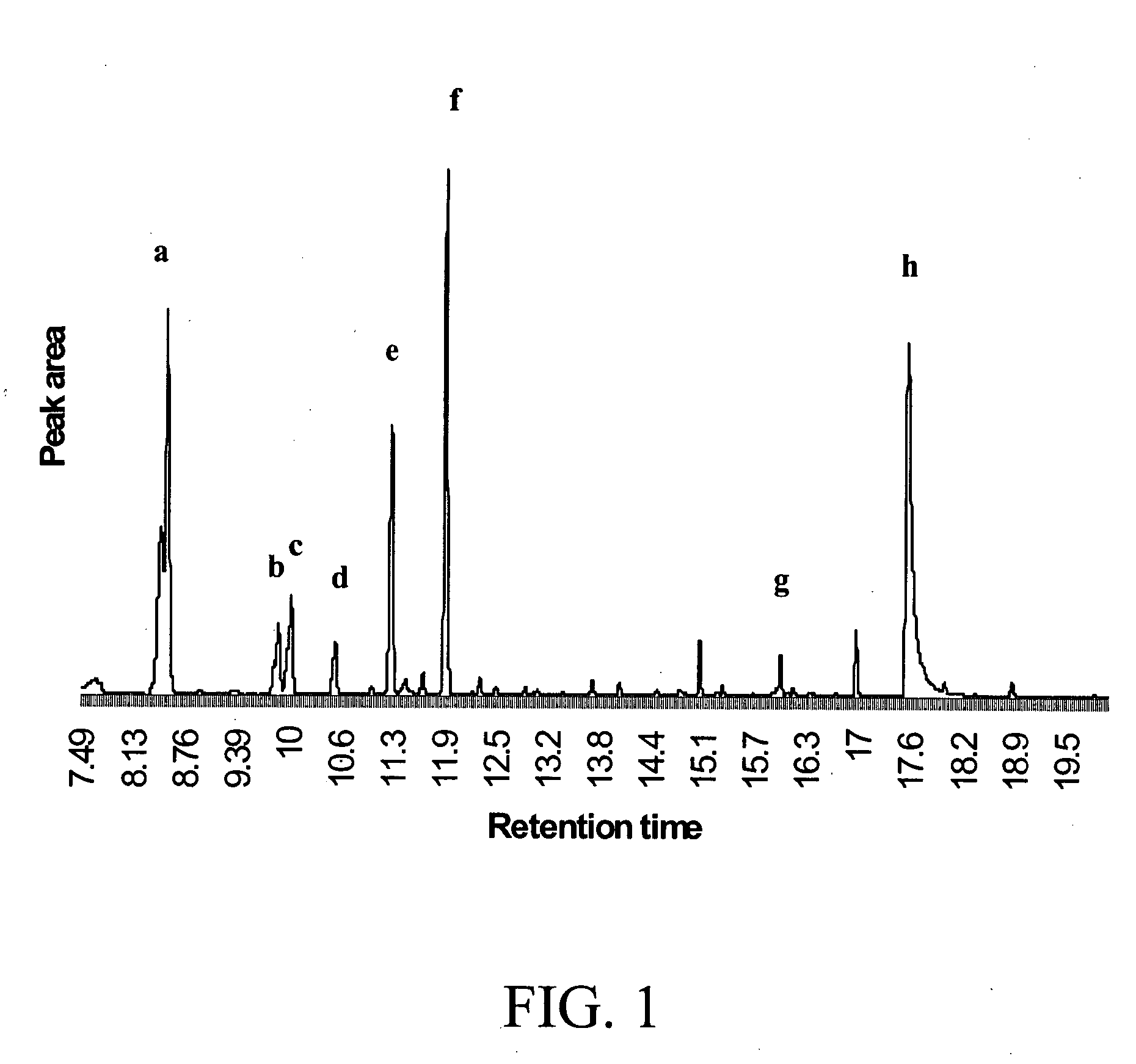
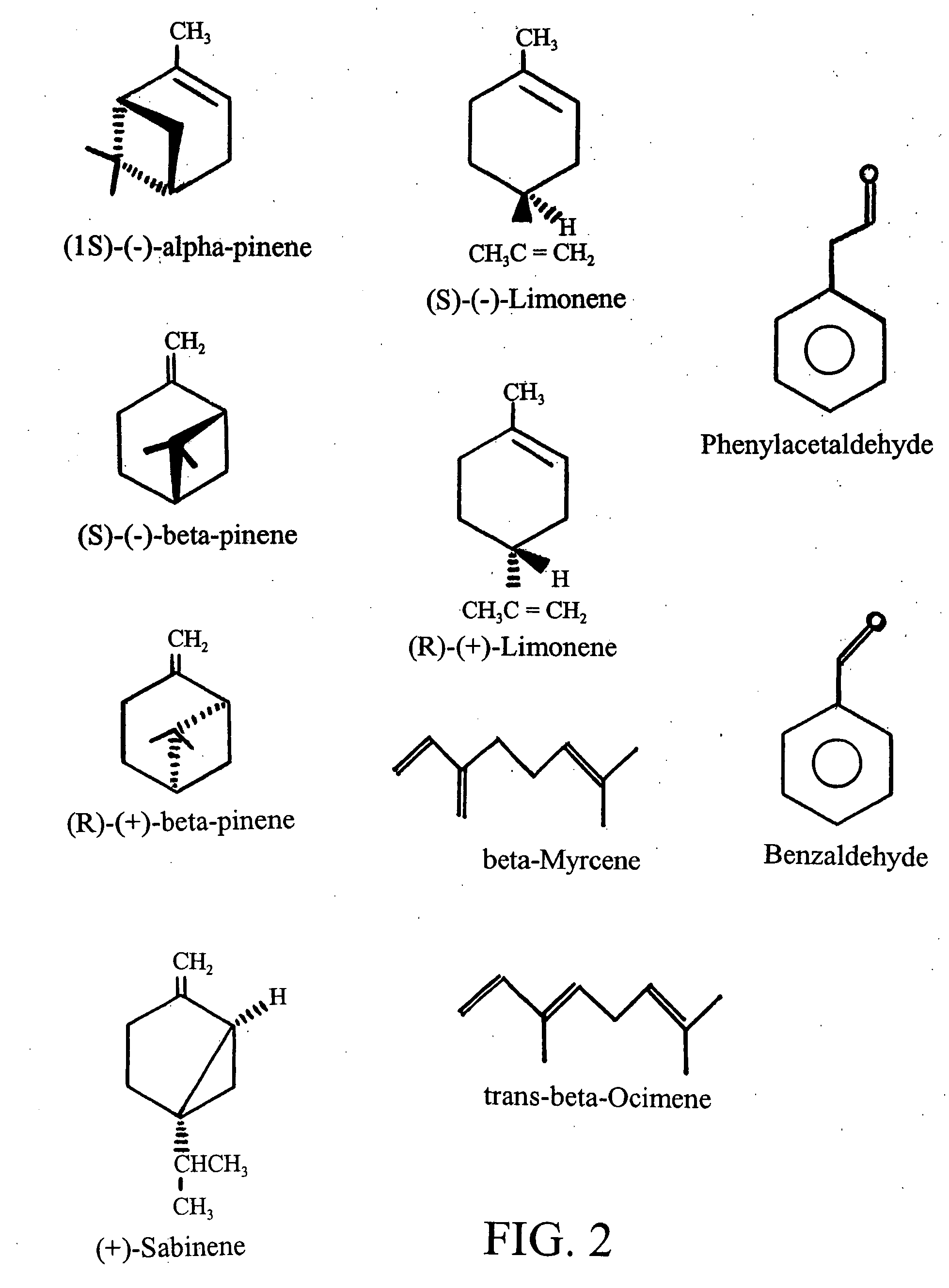
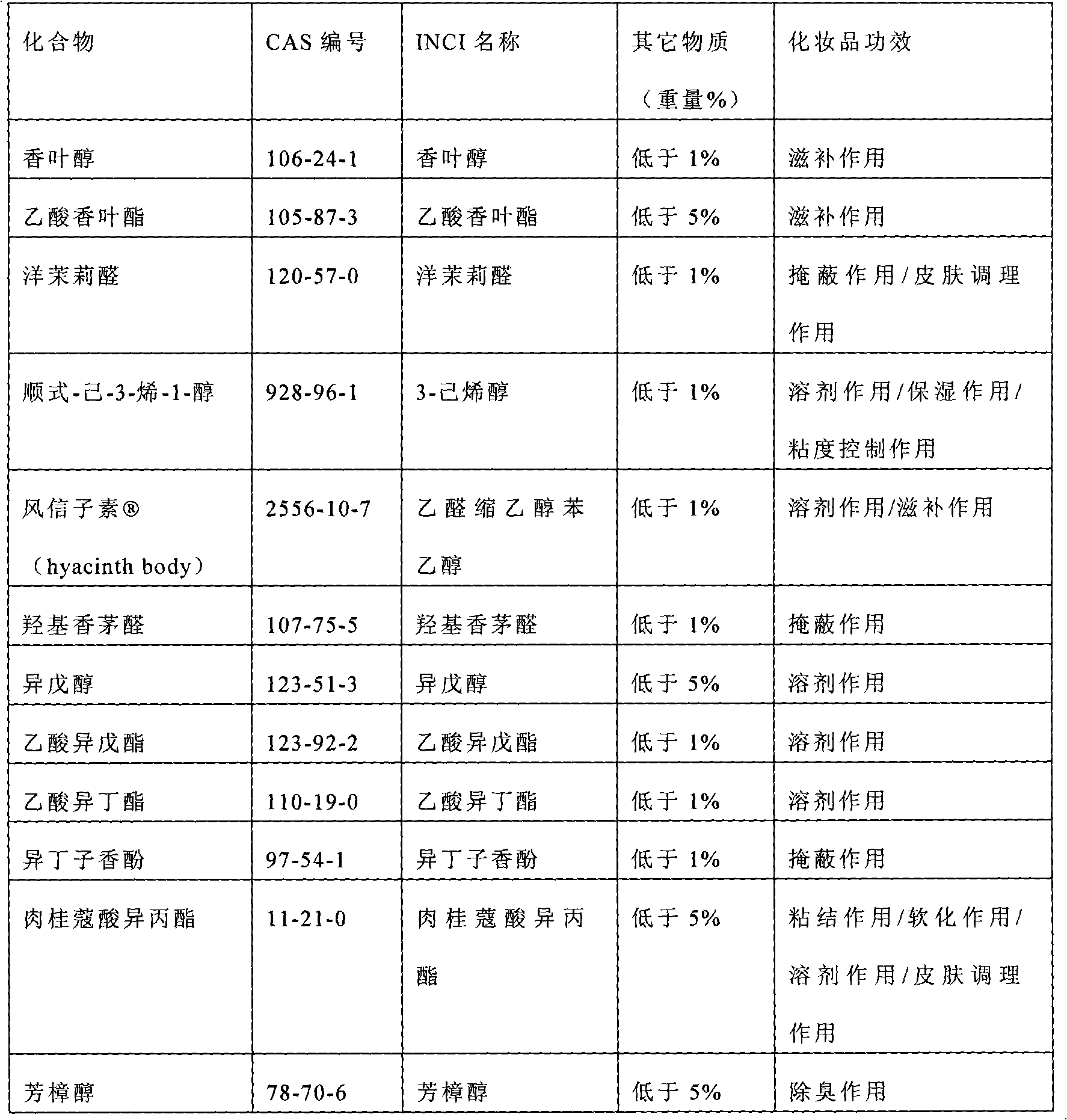
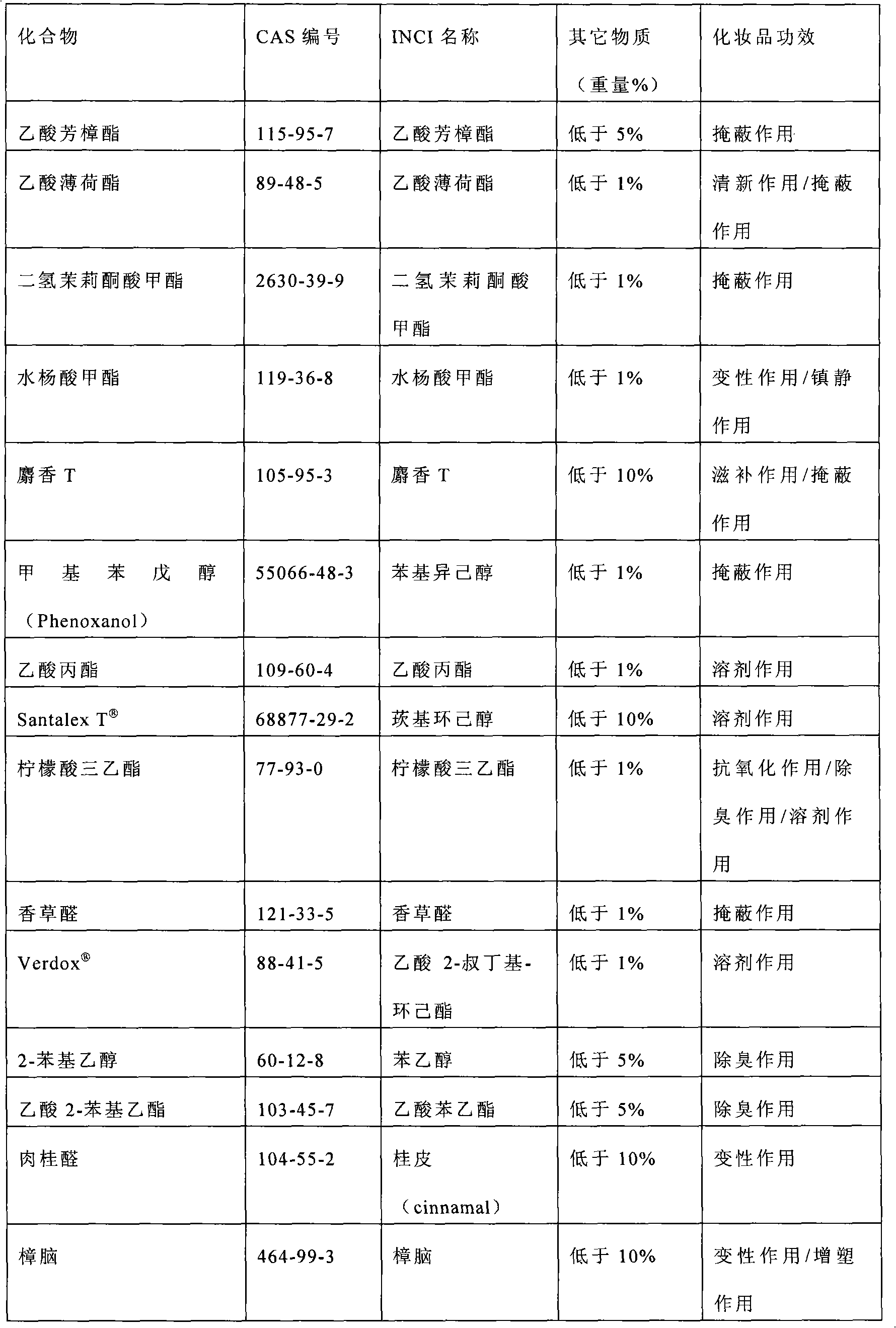
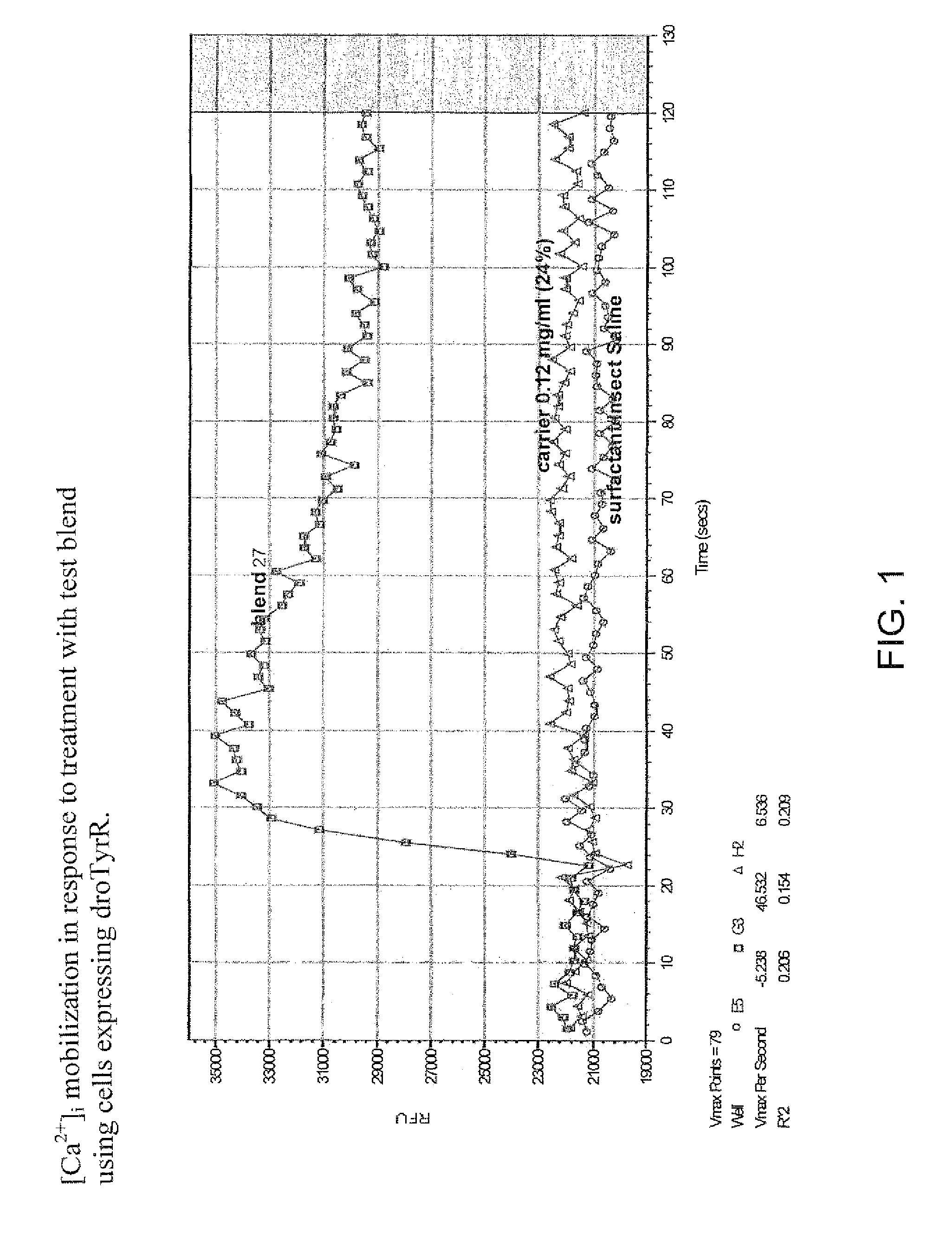
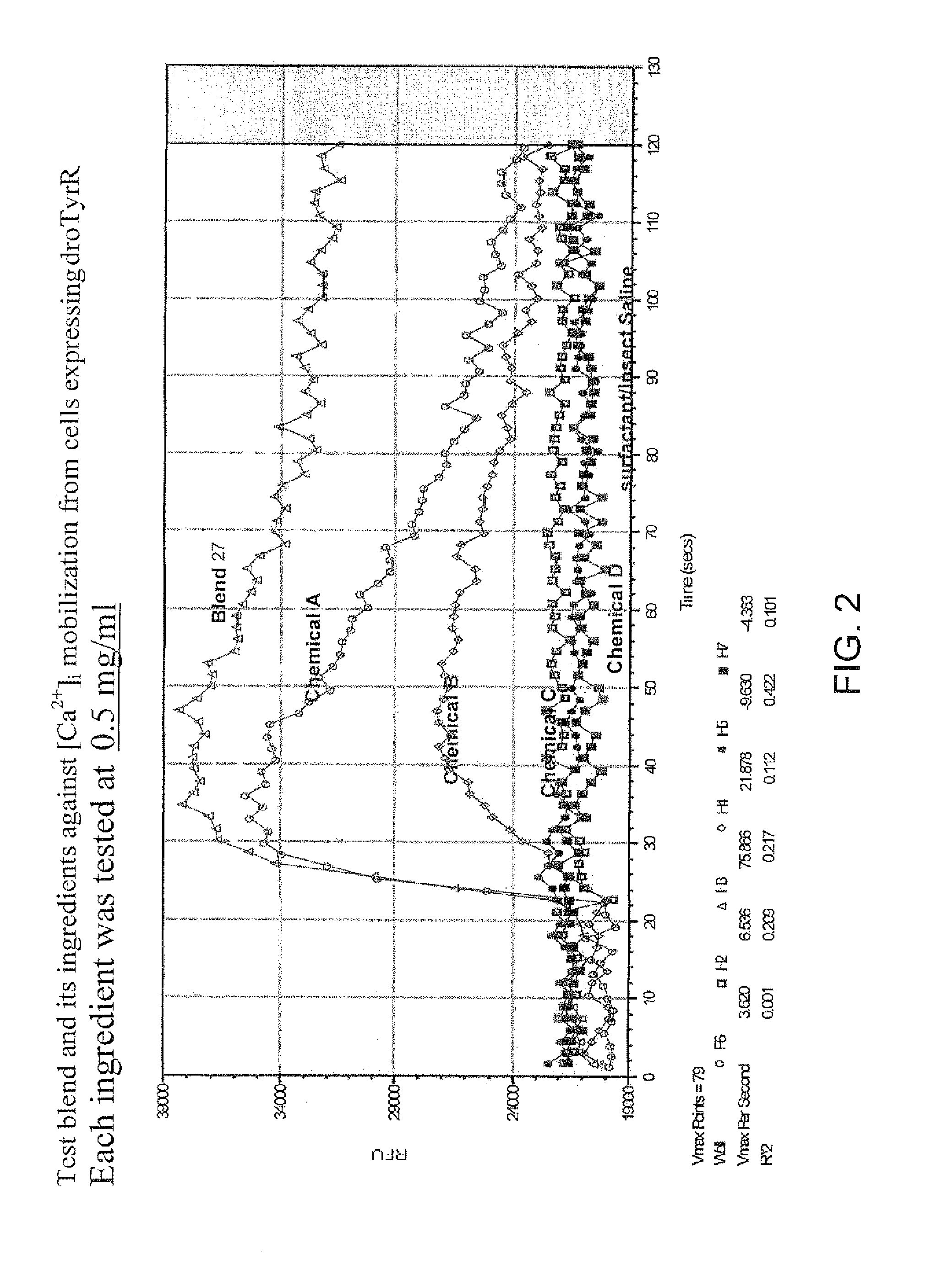
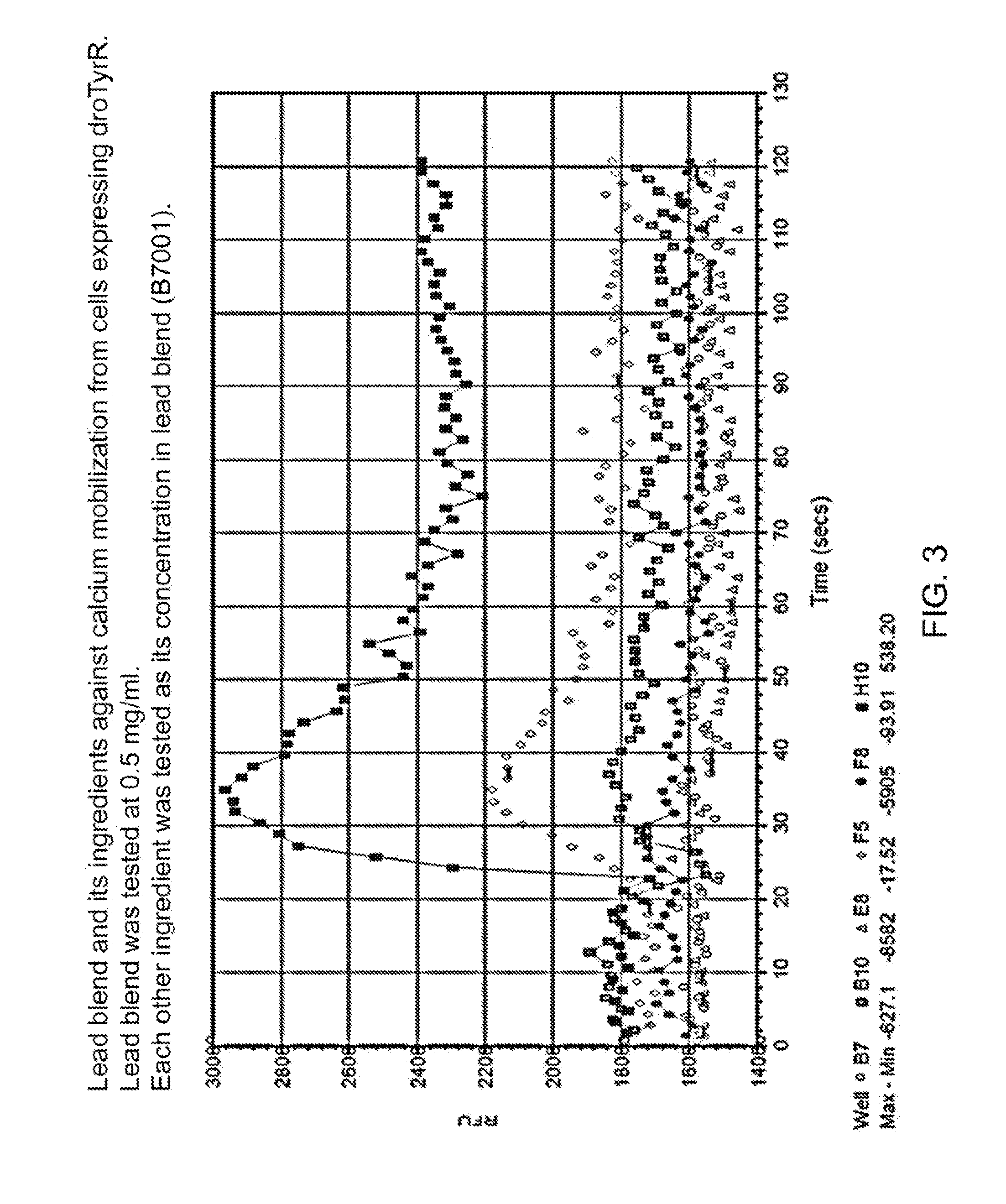
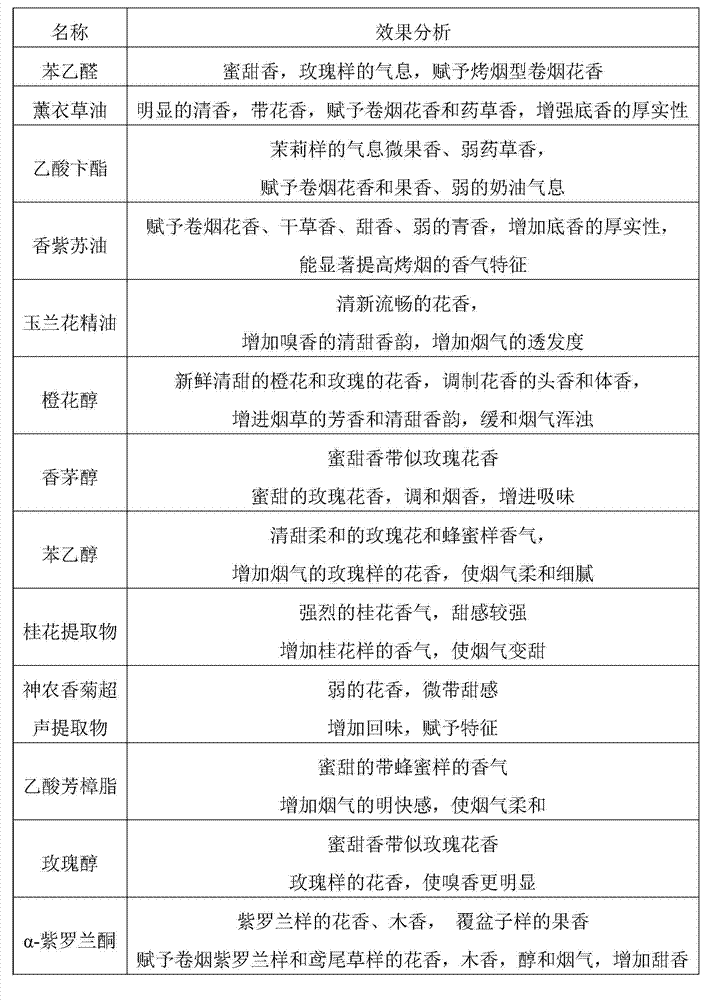
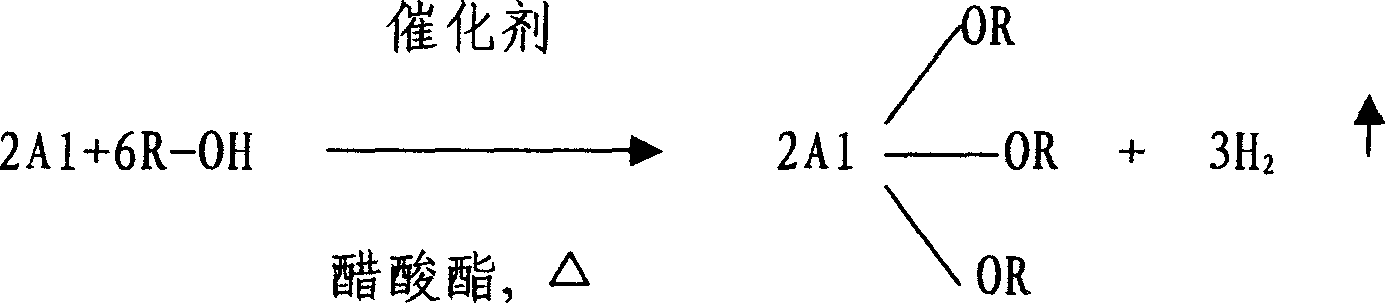Patents
Literature
191 results about "Benzyl acetate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Benzyl acetate is an organic ester with the molecular formula C₉H₁₀O₂. It is formed by the condensation of benzyl alcohol and acetic acid. Similar to most other esters, it possesses a sweet and pleasant aroma, owing to which, it finds applications in personal hygiene and health care products. It is a constituent of jasmin and of the essential oils of ylang-ylang and neroli. It has pleasant sweet aroma reminiscent of jasmine. Further as a flavoring agent it is also used to impart jasmine or apple flavors to various cosmetics and personal care products like lotions, hair creams etc..
Preservative compositions
InactiveUS20080234173A1No additional benefitPromote microbial growthCosmetic preparationsHair cosmeticsEthyl cinnamateBenzaldehyde
The present Invention relates to a preservative composition comprising:a mixture of two to ten of well characterized fragrance raw materials with a cosmetic function, of which at least two are selected from: allyl caproate, benzyl acetate, benzaldehyde, dihydrolsojasmonate, ethyl phenethylacetal, ethyl cinnamate, ethyl methyl phenyl glycidate, ethyl vanillin, 2-heptylcyclopentanone, geranyl acetate, heliotropine, cis-hex-3-en-1-ol, ethylene brassylate, nonalactone gamma, camphylcyclohexanol, undecalactone gamma, 2-t-butylcyclohexylacetate, pentyl salicylate, 2-phenylethanol and 2-phenylethyl acetate; and are contained in at least 20% by weight of said mixture;at least one preservative; and a sequestrant.
Owner:TAKASAGO INTERNATIONAL CORPORATION
Fragrance compositions
InactiveUS20080096790A1Minimally disruptiveEasy to moveCosmetic preparationsToilet preparationsHexyl acetateLemon oil
A method of promoting activated, pleasant moods through the inhalation of energising, non-stressing fragrances (invigorating fragrances) comprising at least 75% by weight, preferably 85% by weight of perfume materials drawn from the following groups:A) At least 10% by weight in total of at least three materials drawn from Group ‘IMP’ comprising: allyl amyl glycolate; benzyl salicylate; bergamot oil; coriander oil; cyclamen aldehyde; 1-(2,6,10-trimethylcyclododeca-2,5,9-trien-1-yl)ethanone; allyl (cyclohexyloxy)acetate; Damascenia 185 SAE; 2,4-dimethylheptan-1-ol; fir balsam; fir needle oil; 3-(4-ethylphenyl)-2,2-dimethylpropanal; ginger oil; guaiacwood; linalyl acetate; litsea cubeba oil; methyl 2,4-dihydroxy-3,6-dimethylbenzoate; nutmeg oil; olibanum oil; orange flower oil; Ozonal AB 7203C; patchouli oil; rose oxide; rosemary oil; sage clary oil; spearmint oil; Tamarine AB 8212E; tarragon oil;B) Optionally up to 90% of materials from the following groups:Group ‘HMR’ comprising:allyl ionone; benzyl acetate; cis-jasmone; citronellol; ethyl linalol; ethylene brassylate; 4-methyl-2-(2-methylpropyl)tetrahydro-2H-pyran-4-ol; geraniol; geranium oil; isoeugenol; lemon oil; 2,4-dimethylcyclohex-3-ene-1-carbaldehyde; 3-(4-hydroxy-4-methylpentyl)cyclohex-3-ene-1-carbaldehyde; 4-(4-hydroxy-4-methylpentyl)cyclohex-3-ene-1-carbaldehyde; alpha-iso-methyl ionone; 3-methylcyclopentadec-2-en-1-one; cyclopentadecanone; cyclohexadecanolide; gamma-undecalactone.Group ‘HMI’ comprising:1-{[2-(1,1 -dimethylethyl)cyclohexyl]oxy}butan-2-ol; 3a,6,6,9a-tetramethyldodecahydronaphtho[2,1 -{b}]furan; alpha-damascone; dihydromyrcenol; eugenol; 3-(1,3-benzodioxol-5-yl)-2-methylpropanal; 2,4-dimethylcyclohex-3-ene-1-carbaldehyde; mandarin oil; orange oil; 2-(1,1-dimethylethyl)cyclohexyl acetate.Group ‘HMP’ comprising:1-(2,6,6,8-tetramethyltricyclo[5.3.1.0 {1,5}]undec-8-en-9-yl)ethanone; allyl cyclohexyl propionate; allyl heptanoate; Apple Oliffac S pcmf; 7-methyl-2H-1,5-benzodioxepin-3(4H)-one; cassis base; cis-3-hexenyl salicylate; damascenone; gamma-decalactone; ethyl acetoacetate; ethyl maltol; ethyl methyl phenylglycidate; hexyl acetate; (3E)-4-methyldec-3-en-5-ol; 2,5,5-trimethyl-6,6-bis(methyloxy)hex-2-ene; 4-(4-hydroxyphenyl)butan-2-one; styrallyl acetate; 2,2,5-trimethyl-5-pentylcyclopentanone; ylang oil. Group ‘RMP’ comprising: anisic aldehyde; (2Z)-2-ethyl-4-(2,2,3-trimethylcyclopent-3-en-1-yl)but-2-en-1-ol; benzoin siam resinoid; ethyl vanillin; oxacyclohexadec-12(13)-en-2-one; hexyl salicylate; hydroxycitronellal; jasmin oil; 3-methyl-5-phenylpentan-1-ol; 2-(phenyloxy)ethyl 2-methylpropanoate; alpha-terpineol; vanillin;Group ‘GEN’ comprising:cyclopentadecanolide; oxacyclohexadecan-2-one; hexyl cinnamic aldehyde; ionone beta; isobornyl cyclohexanol; 1-(2,3,8,8-tetramethyl-1,2,3,4,5,6,7(8),8(8a)-octahydronaphthalen-2-yl)ethanone; 4-(1,1-dimethylethyl)phenyl]-2-methylpropanal; linalol; methyl dihydrojasmonate; 2-phenylethanol;provided the following conditions are met:(a) IMPs>=HMPs+HMRs(b) IMPs+HMIs+GENs>=70%(c) (IMP+HMI) / (IMP+HMI+RMP+HMR)>=0.7(d) IMPs / (HMPs+RMPs+IMPs)>=0.5(e) IMPs / [(HMPs+RMPs+IMPs)+(100−TOTAL)]>=0.3wherein ‘IMPs’ indicates the sum of the percentages of materials within Group IMP, and similarly for the remaining groups, the symbol ‘>=’ indicates ‘at least equal to’, and ‘TOTAL’ is the sum of HMPs, HMRs, HMIs, IMPs, RMPs and GENs, provided also that low odour or no odour solvents are excluded from the calculation of these sums is provided which have an invigorating effect when inhaled by a subject.
Owner:GIVAUDAN NEDERLAND SERVICES
Patterning process
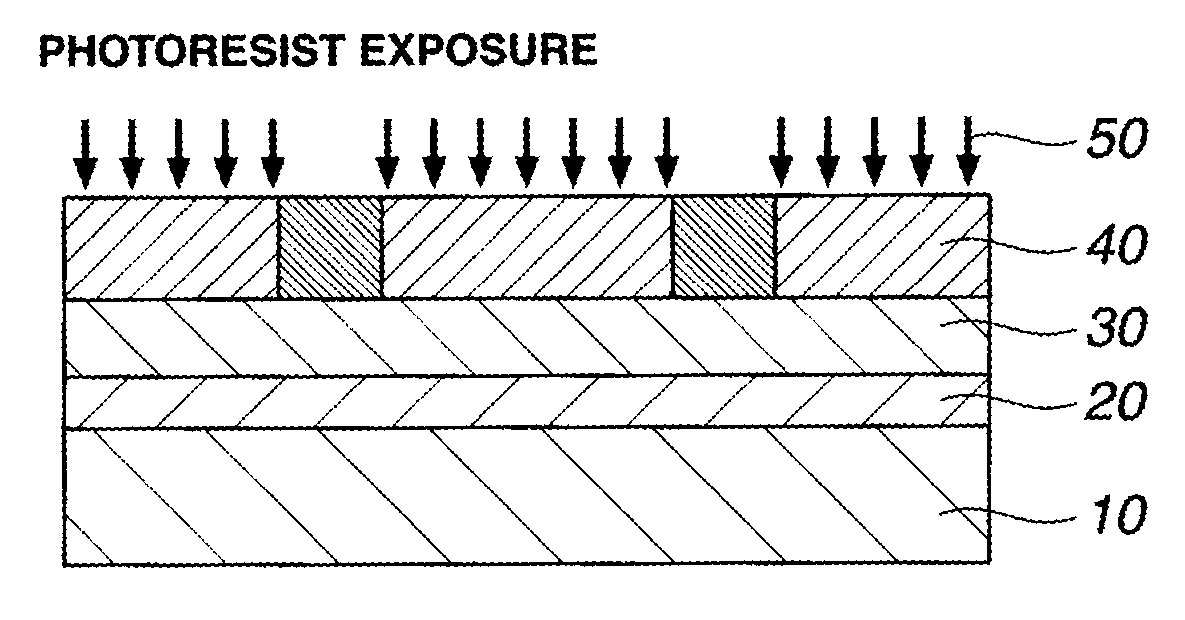
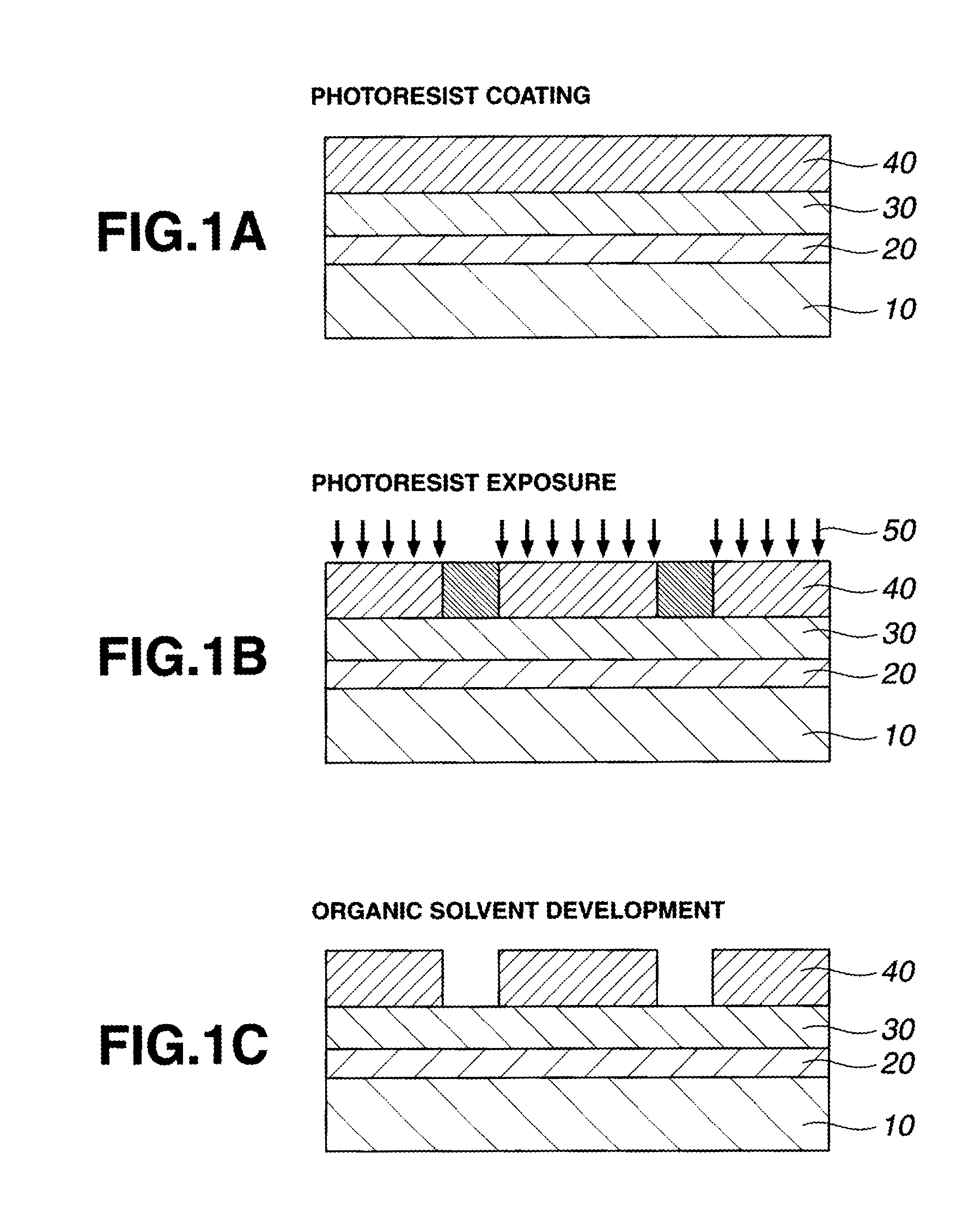
ActiveUS20120009529A1High dissolution contrastIncrease contrastPhotomechanical exposure apparatusMicrolithography exposure apparatusResistMeth-
A pattern is formed by applying a resist composition comprising a (meth)acrylate copolymer comprising both recurring units having an acid labile group-substituted carboxyl group and recurring units having a lactone ring, an acid generator, and an organic solvent onto a substrate, prebaking the composition to form a resist film, exposing the resist film to high-energy radiation, baking, and developing the exposed film with a developer. The developer comprises at least 40 wt % of an organic solvent selected from methyl benzoate, ethyl benzoate, phenyl acetate, benzyl acetate, methyl phenylacetate, benzyl formate, phenylethyl formate, methyl 3-phenylpropionate, benzyl propionate, ethyl phenylacetate, and 2-phenylethyl acetate.
Owner:SHIN ETSU CHEM IND CO LTD
Compound fruit fragrance and flower fragrance essence for daily chemicals and preparation method thereof
InactiveCN105316109AImprove stabilityMeet the needs of material and cultural lifeEssential-oils/perfumesBenzoic acidDamascone
Owner:广东铭康香精香料有限公司
Attractants for moths
InactiveUS20050031661A1Effective attractantReadily apparentBiocideDead animal preservation2-methoxybenzoateDecoy
Compositions and lures are described which provide synthetic chemical attractants which function as highly effective attractants for male, female, or male and female moths, primarily moths of the family Noctuidae. In one aspect, the attractants provide an effective attractant amount of β-myrcene and phenylacetaldehyde or benzyl acetate. In another aspect, the attractants provide an effective attractant amount of phenylacetaldehyde and methyl-2-methoxybenzoate, but do not include methyl salicylate. By attracting moths to traps or baits, the chemical attractants provide a means for detecting, surveying, monitoring, and controlling lepidopteran pests.
Owner:UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF AGRI THE
Preservative compositions
InactiveCN101254157AHave effectHas a cosmetic effectCosmetic preparationsHair cosmeticsEthyl cinnamateBenzaldehyde
Owner:TAKASAGO INTERNATIONAL CORPORATION
Pest control using natural pest control agent blends
Embodiments of the invention relate to a composition for controlling a target pest, wherein the composition includes at least two active ingredients selected from the group consisting of thymyl acetate, linalyl acetate, amyl butyrate, anise star oil, black seed oil, p-cymene, geraniol, isopropyl myristate, d-limonene, linalool, lilac flower oil, methyl salicylate, alpha-pinene, piperonal, piperonyl alcohol, tetrahydrolinalool, thyme oil white, thyme oil red, thymol, vanillin, and winter-green oil, wherein the composition causes synergistic control of the target pest.
Owner:TYRATECH +1
Flower fragrance aroma essence for tobacco
InactiveCN102827693AAvoid withEasy development workTobacco preparationEssential-oils/perfumesPerilla oilPollen
The invention discloses flower fragrance aroma essence for tobacco, which is mixed by the following raw material components by weight: 0.2-0.3 part of phenylacetaldehyde, 1-2 parts of lavender oil, 0.6-0.8 part of benzyl acetate, 5-8 parts of fragrant perilla oil, 1-5 parts of magnolia essential oil, 1-5 parts of nerol, 2-5 parts of citronellol, 1-3 parts of phenylethyl alcohol, 0.05-0.2 part of sweet osmanthus extracts, 1-3 parts of dendranthema indicum ultrasonic extracts, 0.6-0.8 part of linalyl acetate, 1-5 parts of rhodinol, 2-3 parts of alpha-ionone, and 58.9-83.5 parts of 95% ethanol. The invention prevents pollen gas of the flower extracts by extraction of various flowers. The invention realizes effective compounding of single raw materials, which is a scientific and effective method. The flower fragrance aroma essence for tobacco is blended by raw materials with flower fragrance aroma, and a proper amount of the essence can be added directly into surface fragrance essence for tobacco. The essence effect is improved, and convenience for essence development is provided; the cost is low, and the usage is convenient.
Owner:HUBEI CHINA TOBACCO IND +1
Mosquito repellent incense containing plant vinegar liquid and use thereof
ActiveCN101297644AAddressing drug resistanceSufficient sourceBiocideArthropodicidesPolyvinyl alcoholBULK ACTIVE INGREDIENT
The invention relates to an anti-mosquito incense containing plant vinegar and application thereof, belonging to the sanitary insecticide article technical field. When made into a solid sheet type, the anti-mosquito incense comprises 45 to 65 percent of plant vinegar, 35 to 55 percent of binder and 0.1 to 1.0 percent of essence; when made into a liquid type, the anti-mosquito incense comprises 90 to 99 percent of plant vinegar, and 4 to 8 percent of essence. The plant vinegar is one or a plurality of bamboo vinegar, wood vinegar and / or grass vinegar; the binder is carbon powder, flour, corn starch, polyvinyl alcohol, WX-602 gelatine powder produced by Juxiang (a name of a company) and sticky wood powder; the essence is one or a plurality of benzyl acetate, citric acid, wintergreen oil, dodecanoic acid, Australian orange essence oil, 2, 3-diacetyl propyl lauric acid ester, menthol and citronella oil. The anti-mosquito incense adopts plant vinegar as the active ingredient, has three functions of killing mosquito, killing fly and killing cockroach with good control effect and solves the problem that the using of chemical anti-mosquito incense can cause the insect to generate resistance to drugs.
Owner:杭州银帆环境科技有限公司
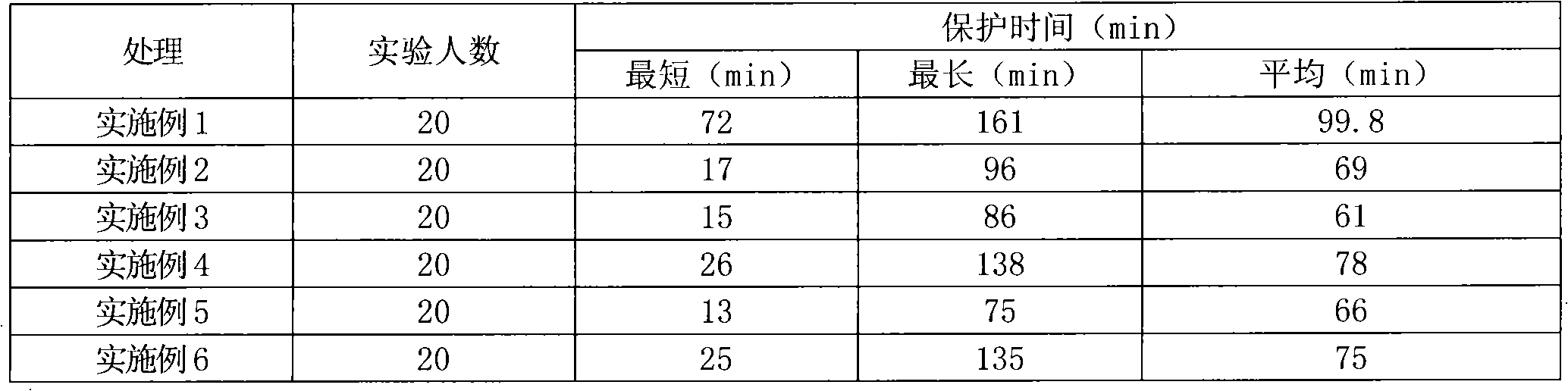
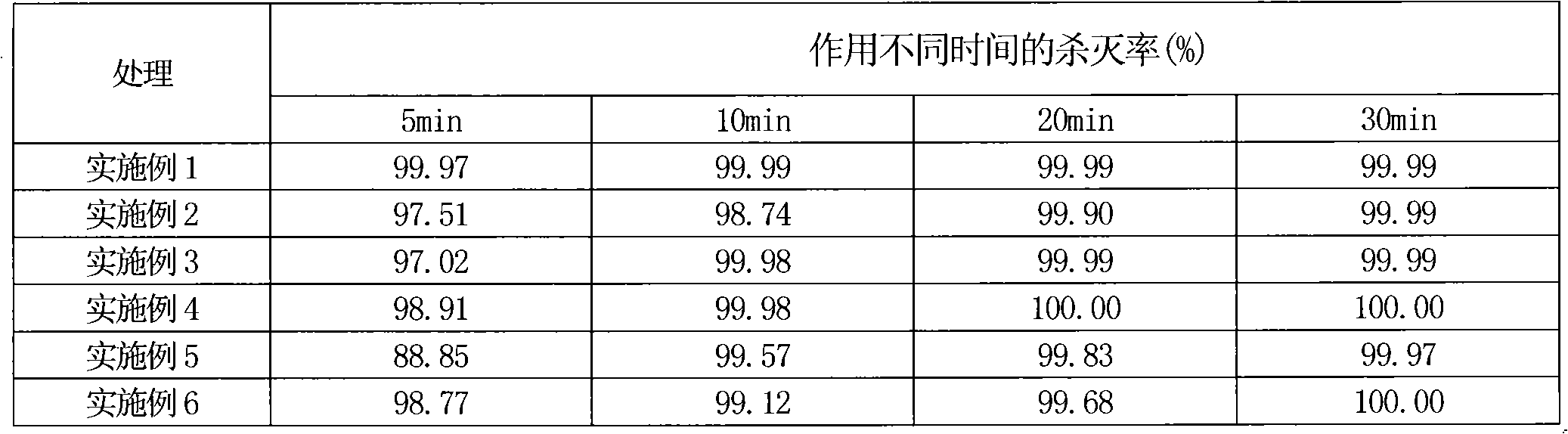
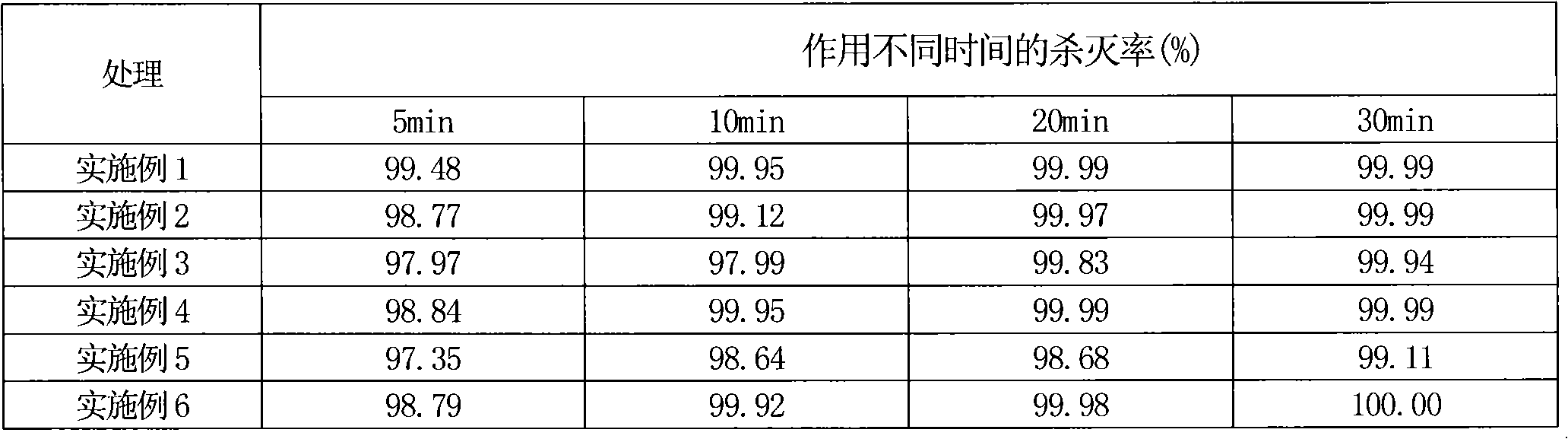
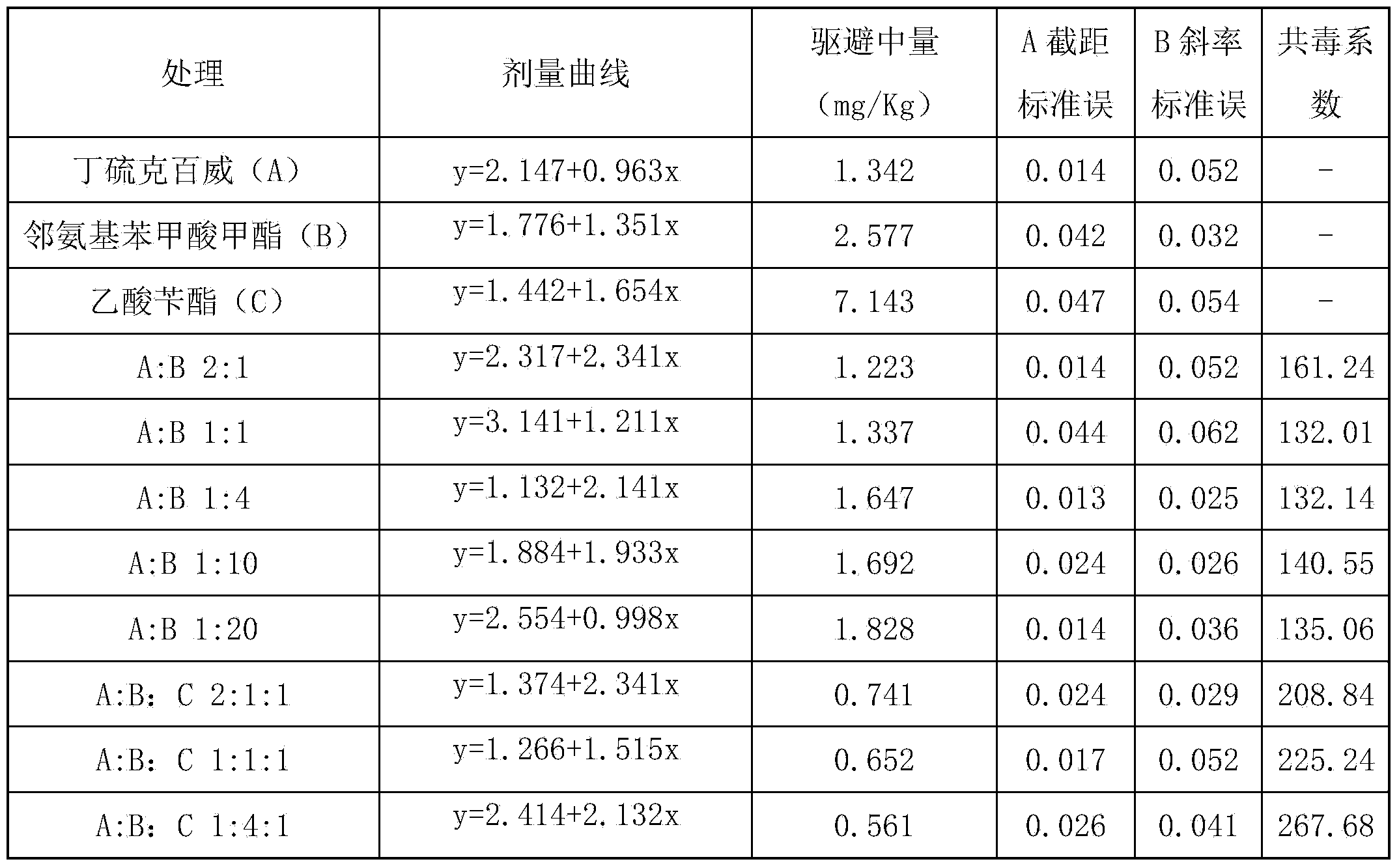
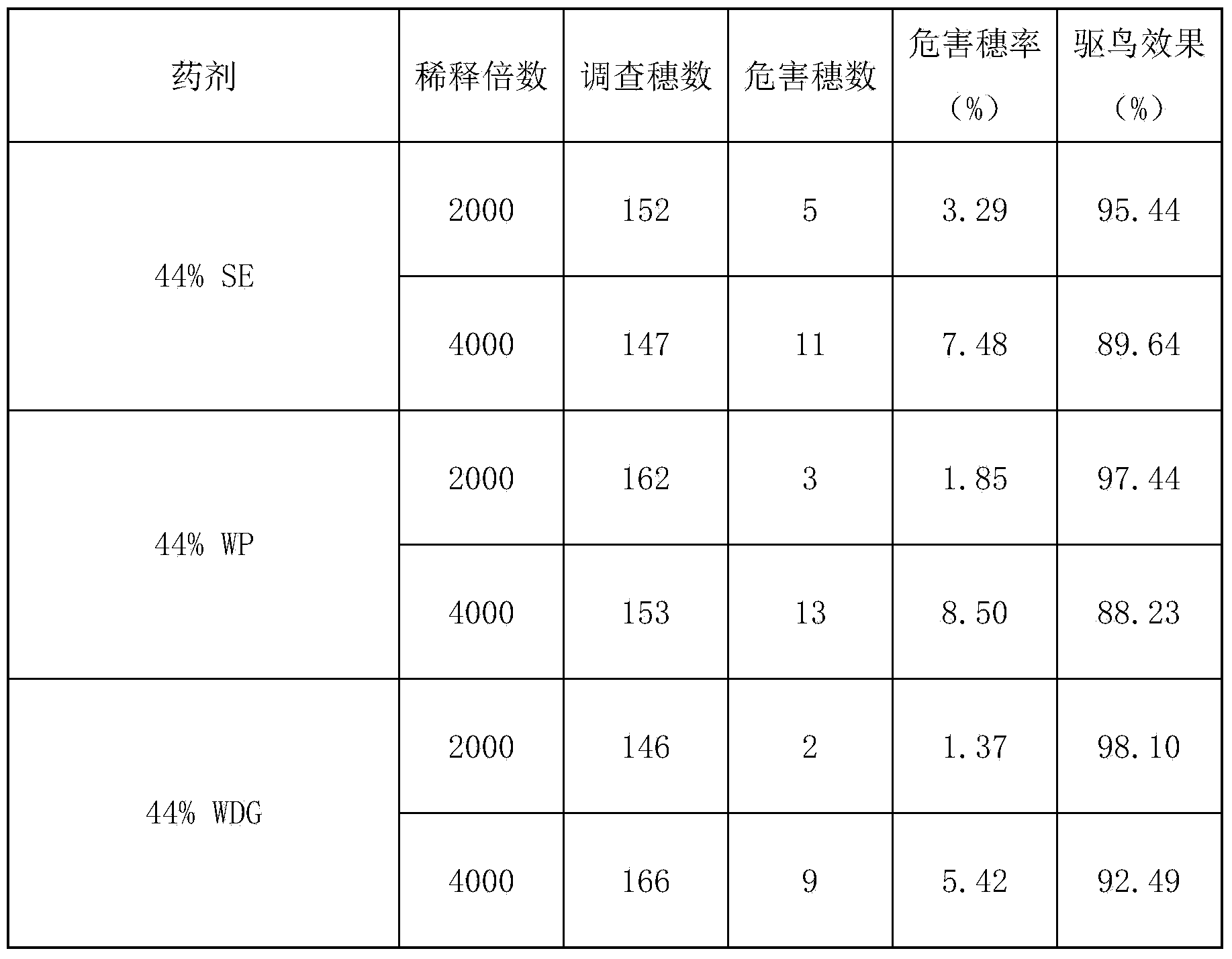
Compound bird repellent containing carbosulfan, methyl ortho-aminobenzoate and benzyl acetate
The invention provides a compound bird repellent containing carbosulfan, methyl ortho-aminobenzoate and benzyl acetate. The compound bird repellent is prepared by mixing the three active ingredients including the carbosulfan, the methyl ortho-aminobenzoate and the benzyl acetate in proportion, wherein the mass ratio of the carbosulfan to the methyl ortho-aminobenzoate is (2 to 1)-(1 to 20), and the mass ratio of the methyl ortho-aminobenzoate to the benzyl acetate is (1 to 1)-(1 to 2). After the compound bird repellent is used, the harm such as seedling shortage, damage to fruits and grains at the harvest time and the like caused by mass feeding of birds to the field crops, orchards and the like can be effectively avoided.
Owner:ANHUI AGRICULTURAL UNIVERSITY
Grassland spodoptera litura adult attractant and trapping device containing attractant
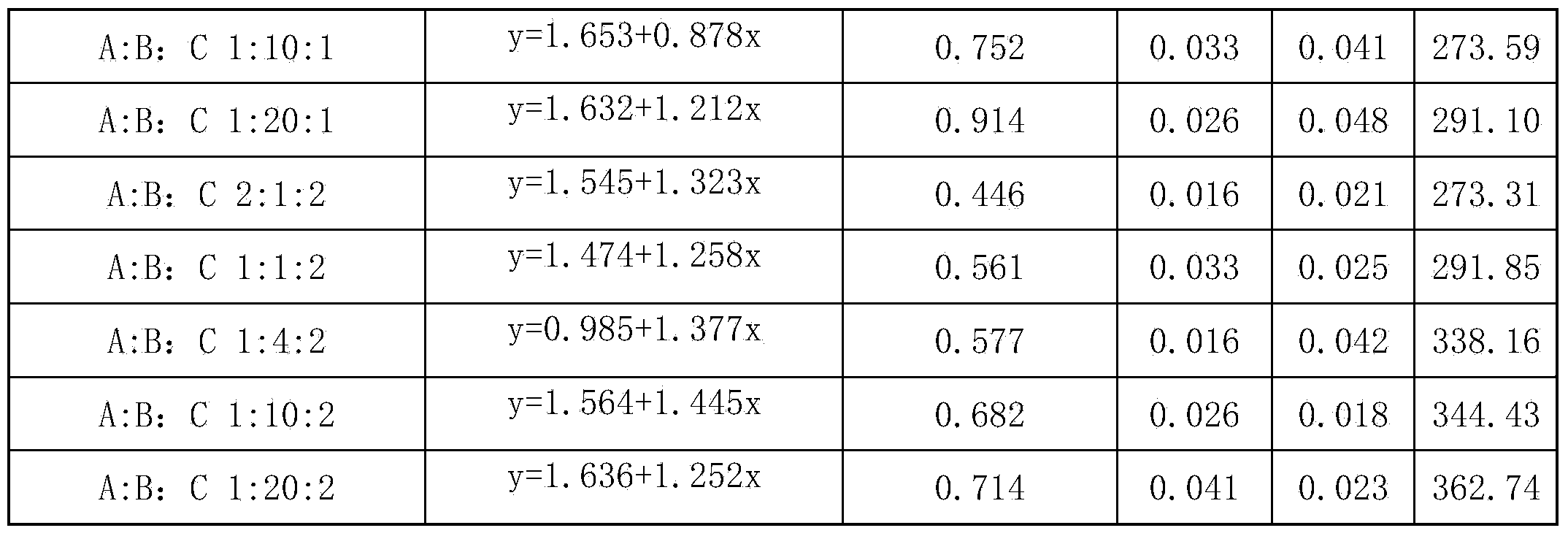
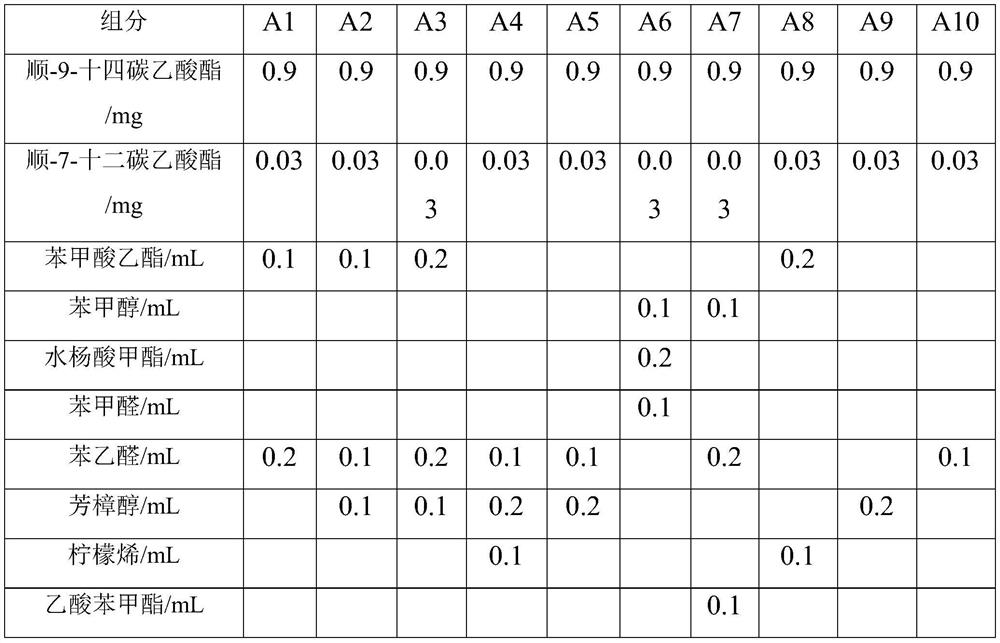
ActiveCN111685118AGood lure effectSolve the difficulty that is hard to lureBiocidePest attractantsBenzoic acidDodecane
The invention relates to a grassland spodoptera litura adult attractant based on a plant volatile matter and a trapping device comprising the grassland spodoptera litura adult attractant. The grassland spodoptera litura adult attractant is composed of a sex attractant and the plant smell volatile matter. The sex attractant is prepared from cis-9-tetradecane acetate and cis-7-dodecane acetate; theplant smell volatile matter is one or more of ethyl benzoate, benzyl alcohol, methyl salicylate, benzaldehyde, phenylacetaldehyde, limonene, linalool and benzyl acetate. The adult attractant for the grassland spodoptera litura has a good attracting effect on the grassland spodoptera litura through synergistic interaction of the sex attractant and the plant smell volatile matter, can attract part of female insects, can be used for monitoring and large-area prevention and control of the grassland spodoptera litura, and has a good application prospect.
Owner:漳州市英格尔农业科技有限公司
Blueberry essence for cigarette paper
InactiveCN106398868AStrong noveltyObvious blueberry aromaTobacco preparationEssential-oils/perfumesBlueberry extractAlcohol
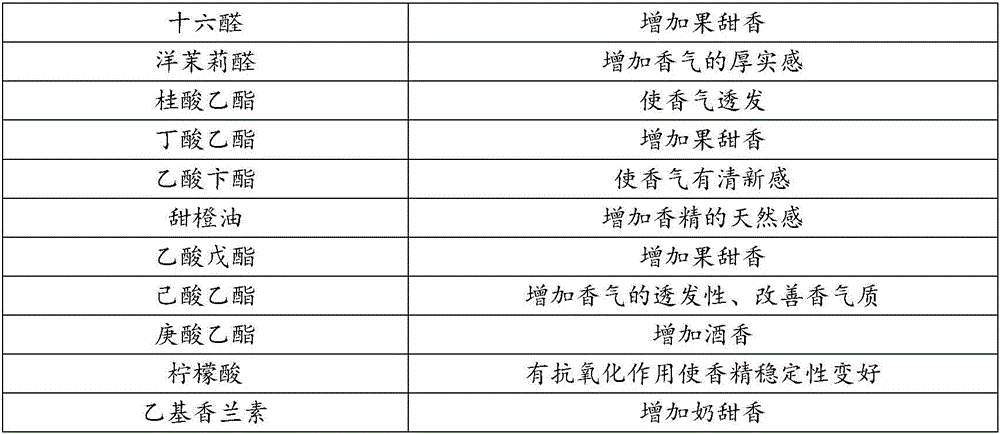
The invention provides blueberry essence for cigarette paper. The blueberry essence is prepared from, by weight, 1-2 parts of blueberry essence and 8-9 parts of blueberry extract, the blueberry essence is prepared by mixing, by mass, 1%-2% of beta-ionone, 1%-2% of alpha-ionone, 5%-10% of hexadecanal, 10%-15% of heliotropin, 3%-5% of ethyl cinnamate, 8%-10% of ethyl butyrate, 8%-10% of benzyl acetate, 3%-5% of sweet orange oil, 1%-3% of amyl acetate, 1%-2% of ethyl hexanoate, 1%-2% of ethyl oenanthate, 1%-3% of citric acid, 3%-5% of ethyl vanillin and 26%-54% of 95% ethyl alcohol. The blueberry extract is extracted through a supercritical extraction process and has aroma components and natural anthocyanin of blueberries. The blueberry essence is added into the cigarette paper, so that cigarettes have obvious blueberry fragrance and meanwhile have the distinctive blue of the blueberries.
Owner:HUBEI CHINA TOBACCO IND
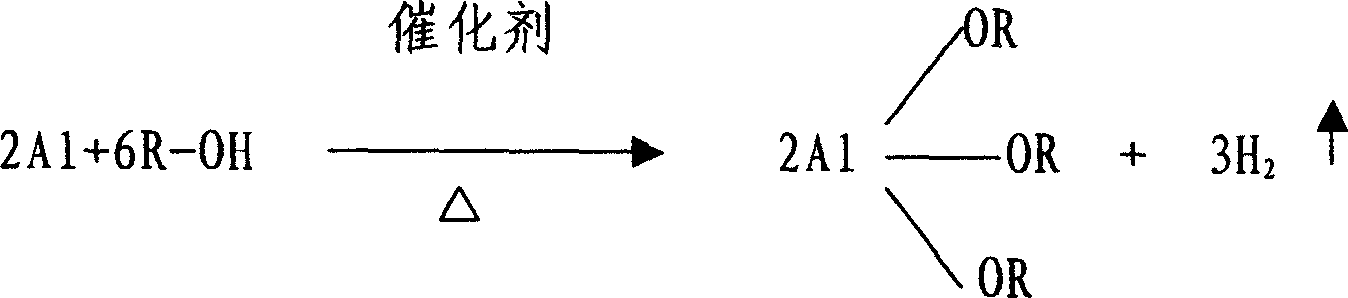
Method for fabricating aluminum alkoxide
A process for preparing alcoholic aluminum by alcohol and aluminum incldues such steps as heat refluxnig alcohol, aluminum and initiator in acetic ester solvent to get alcoholic aluminum acetic ester solution, separating and vaporization to get alcoholic aluminum (the ratio between alcohol and acetic aster is 1:2-3, aluminum add overdose). The acetic ester is Methyl acetate, acetic ether, benzyl acetate, isopropyl acetate or butyl acetate, aluminum is aluminum granule or aluminum crumbs or aluminum powder, its perfidy is higher than 99%.
Owner:ZHEJIANG YUDA CHEM IND
Lemon essence for water-based ink and preparation method of lemon essence
The invention relates to lemon essence for water-based ink. The lemon essence is prepared from, by weight, 1%-30% of lemon essence, 5%-55% of compound modification oligosaccharide and deionized water. The lemon essence is composed of lemon oil, litsea cubeba oil, citral diethyl acetal, myrac aldehyde, bergamot aldehyde, lemonile, decanal, caprylic aldehyde, dodecanenitrile, citonellye nitrile, ethyl methylphenylglycidate, linalyl acetate, linalool, lilial, benzyl acetate, cyclamen aldehyde, terpilenol, terpinyl acetate, anisic aldehyde, verdyl acetate, geraniol, geranyl acetate, citronellol, citronellyl acetate, isocyclocitral, styralyl acetate, isoeugenol, ionone, heliotropin, iso-longitolanone, HHCB and ethyl alcohol. The invention further provides a preparation method of the lemon essence for the water-based ink. According to the lemon essence for the water-based ink and the preparation method of the lemon essence, the compound modification oligosaccharide is used as a wall material, the lemon essence is wrapped so that lemon nanocapsule slow-release essence can be formed, and therefore fragrance depositing and water solubility of the essence are improved.
Owner:SHANGHAI INST OF TECH
Process for the liquid phase oxidation of toluene to benzaldehyde
InactiveUS20070213566A1Reduce formationHigh selectivityOrganic compound preparationKetenes preparationBenzoic acidBenzaldehyde
The present invention provides a liquid phase oxidation of toluene by using catalyst containing manganese (Manganese II acetate) in presence of Lewis acid (Stannous (II) chloride) and a bromide promoter (NaBr), at a temperature of 120° C. and at a pressure of air in the range of 70-400 psig, in presence of carboxylic acid (acetic acid), as a solvent, to obtain high selectivity to benzaldehyde (76%). High activity was obtained with minimum byproducts such as benzoic acid, benzyl alcohol and benzyl acetate.
Owner:COUNCIL OF SCI & IND RES
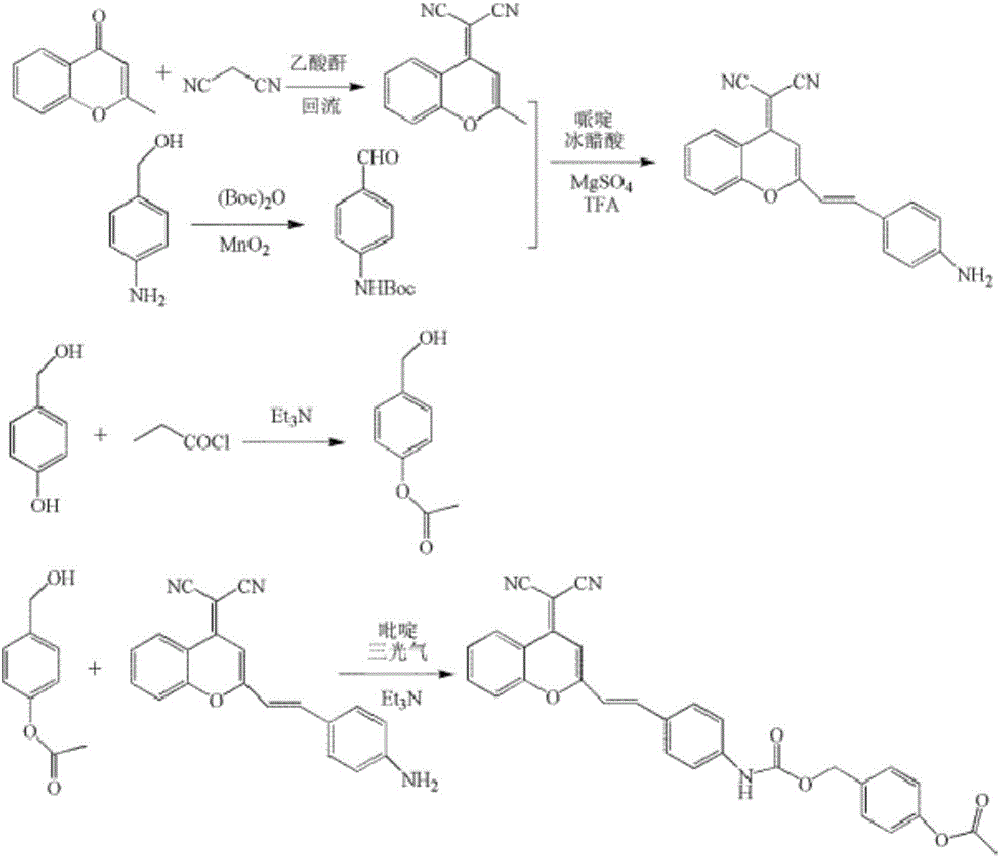
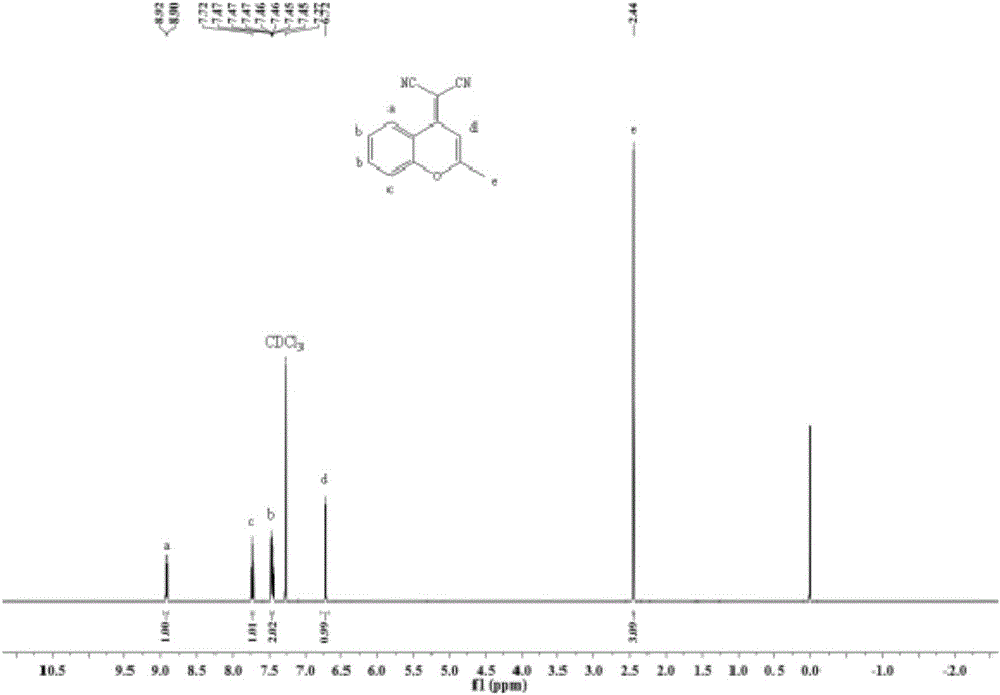
Fluorescent probe used for detecting carboxylesterase and preparation method and application thereof
InactiveCN106478576AImprove anti-interference abilityFluorescent signal does not increaseOrganic chemistryFluorescence/phosphorescenceBenzopyranFluorescence
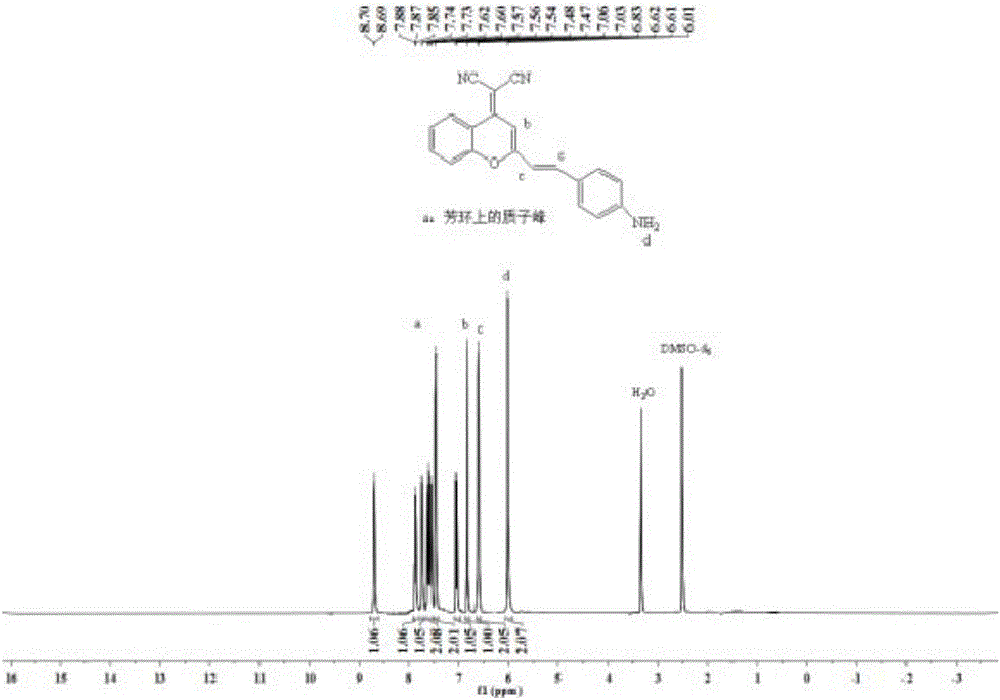
The invention belongs to the technical field of analysis and detection, and discloses a fluorescent probe used for detecting carboxylesterase and a preparation method and an application thereof. The fluorescent probe is 2-{2-[4-(4'-benzyl acetate benzyloxy-formamido)-styryl]-4H-benzopyran-4-yl}-malononitrile, and has a structural formula in a formula (I). and the fluorescent probe is used for detecting carboxylesterase. Compared with the current detection technique of fluorescence, the fluorescent probe has high selective response for carboxylesterase, strong anti-interference performance, and effective and visual detection mode, low investment cost, and simple synthesis route and method, and is suitable for enlarge production and practical application.
Owner:SOUTH CHINA UNIV OF TECH
Thick film green sheet slurry, production method of thick film green sheet slurry, production method of thick film green sheet and production methods of thick film green sheet and electronic device
InactiveUS20090078358A1Strict managementCutting adhesivenessStacked capacitorsCeramic layered productsSolubilityAlcohol
A thick film green sheet slurry, a production method of thick film green sheet slurry, a production method of a thick film green sheet, a thick film green sheet and a production method of an electronic device are provided; by which coating of a relatively thick film becomes possible, a sheet formed after coating has excellent cutting property (strength capable of being cut), and a sheet having high air permeability, excellent handleability and a high adhesive force can be formed. In the present invention, a thick film green sheet slurry comprises a ceramic powder, a binder resin including a butyral based resin as the main component, and a solvent: wherein the solvent includes a good solvent for letting the binder resin dissolved well therein and a poor solvent having lower solubility to the binder resin comparing with that of the good solvent; and the poor solvent is included in a range of 30 to 60 wt % with respect to the entire solvent. The good solvent is alcohol, and the poor solvent may be toluene, xylene, mineral spirit, benzyl acetate, solvent naphtha, etc.
Owner:TDK CORPARATION
Application of shiliangcha extract in preparing medicine
InactiveCN1931200AAvoid irritationSignificant effectDigestive systemPlant ingredientsDiseaseRespiratory tract disease
The present invention relates to the new use of Shiliangcha extract in preparing medicine. Shiliangcha extract is mainly volatile oil prepared with the leaves of calycanthaceae plants, and with the main components including 1.8-cineole, broneol, linalool, benzyl alcohol, benzyl acetate, farnesol, terpineol, 1H-indole, etc. Pharmacological test shows that Shiliangcha extract has obvious effects of inhibiting diarrhea, stress ulcer and enteric hyperanacinesia, and lowering the gastric secretion and total acid displacement. It has high curative effect on abdominal mass and has also excellent curative effect on respiratory tract diseases.
Owner:刘忠达
Method for preparing benzyl acetate by using waste reaction liquid generated in acetate production
InactiveCN103896771AReduce manufacturing costHigh purityOrganic compound preparationCarboxylic acid esters separation/purificationAcetic acidSodium acetate
The invention relates to the technical field of benzyl acetate synthesis and in particular relates to a method for preparing benzyl acetate by using waste reaction liquid generated in acetate production. Benzyl acetate is prepared by steps of treating the waste reaction liquid, performing acid-base reaction, performing esterification reaction, desalting and rectifying. According to the method, benzyl acetate is prepared from acetic acid in the waste reaction liquid during the acetate production, so that the waste resources can be fully utilized, the resource waste can be reduced, and the environmental pollution caused by the acetate production can be alleviated; in addition, as a raw material is the waste reaction liquid generated in the acetate production, the production cost of benzyl acetate can be greatly reduced and the development requirements of environment-friendly and low-cost synthetic routes can be met; in a process of converting the waste reaction liquid into a reactant, heat emitted in the reaction between acetic acid and sodium hydroxide is fully utilized, the energy consumed for heating a sodium acetate solution is reduced, and the purposes of energy conservation and environmental protection are reached; benzyl acetate with the purity higher than 98% still can be prepared from the waste reaction liquid, and the product quality is ensured.
Owner:ZHUHAI FEIYANG NOVEL MATERIALS
Sensitive Skin Perfumes
The invention relates to a fragrance composition for use in cosmetic, toiletry, personal care and cleansing, household cleaning and laundry products which comprises two to ten well characterized fragrance materials having a cosmetic function, of which at least two are selected from: allyl caproate, benzyl acetate, benzaldehyde, dihydroisojasmonate, ethyl phenethylacetal, ethyl cinnamate, ethyl methyl phenyl glycidate, ethyl vanillin, 2-heptylcyclopentanone, geranyl acetate, heliotropine, cis-hex-3-en-1-ol, ethylene brassylate, nonalactone gamma, camphylcyclohexanol, undecalactone gamma, 2-t-butylcyclohexylacetate, pentyl salicylate, 2-phenylethanol, hinokitiol and 2-phenylethyl acetate, and comprises at least 20% by weight of the composition.
Owner:TAKASAGO INTERNATIONAL CORPORATION
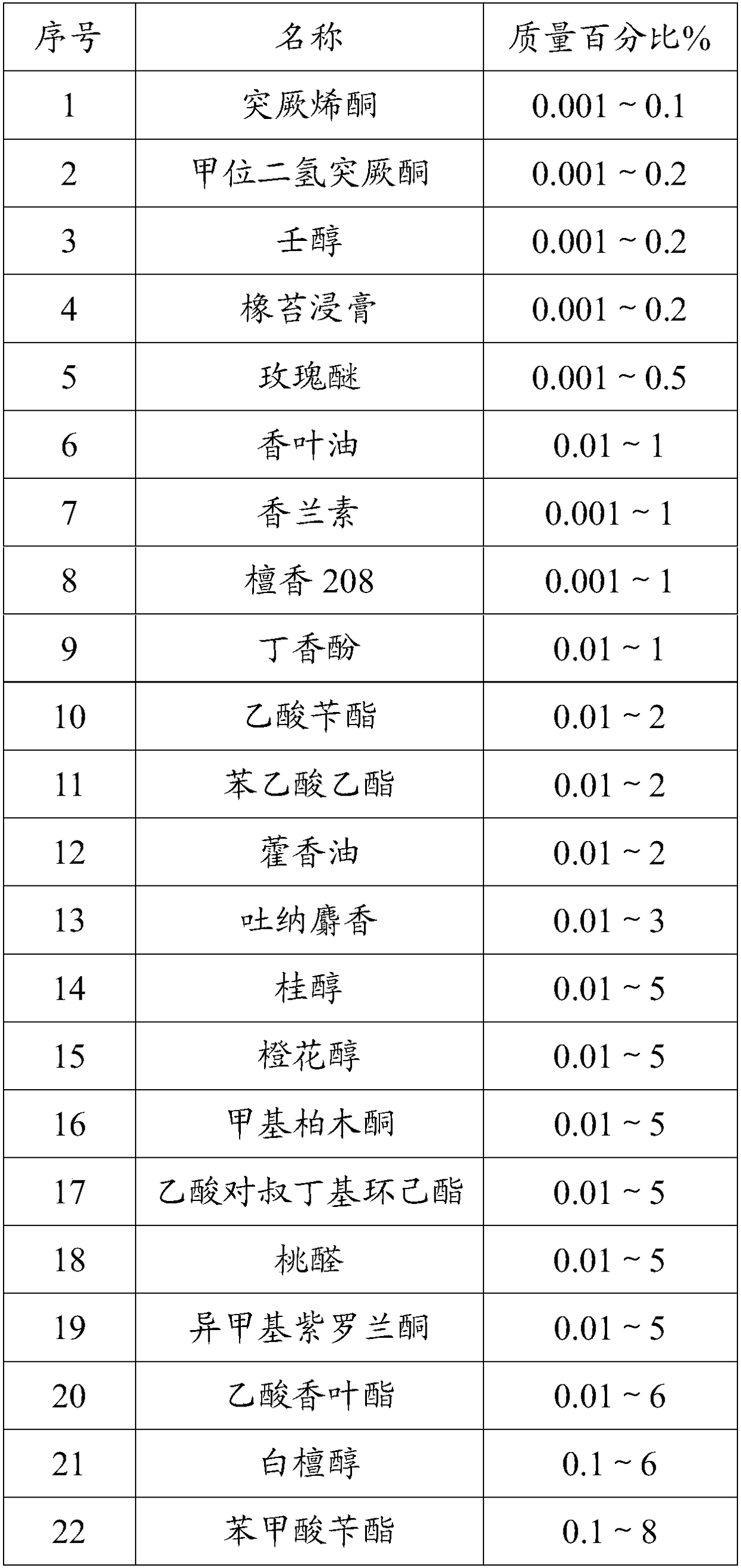
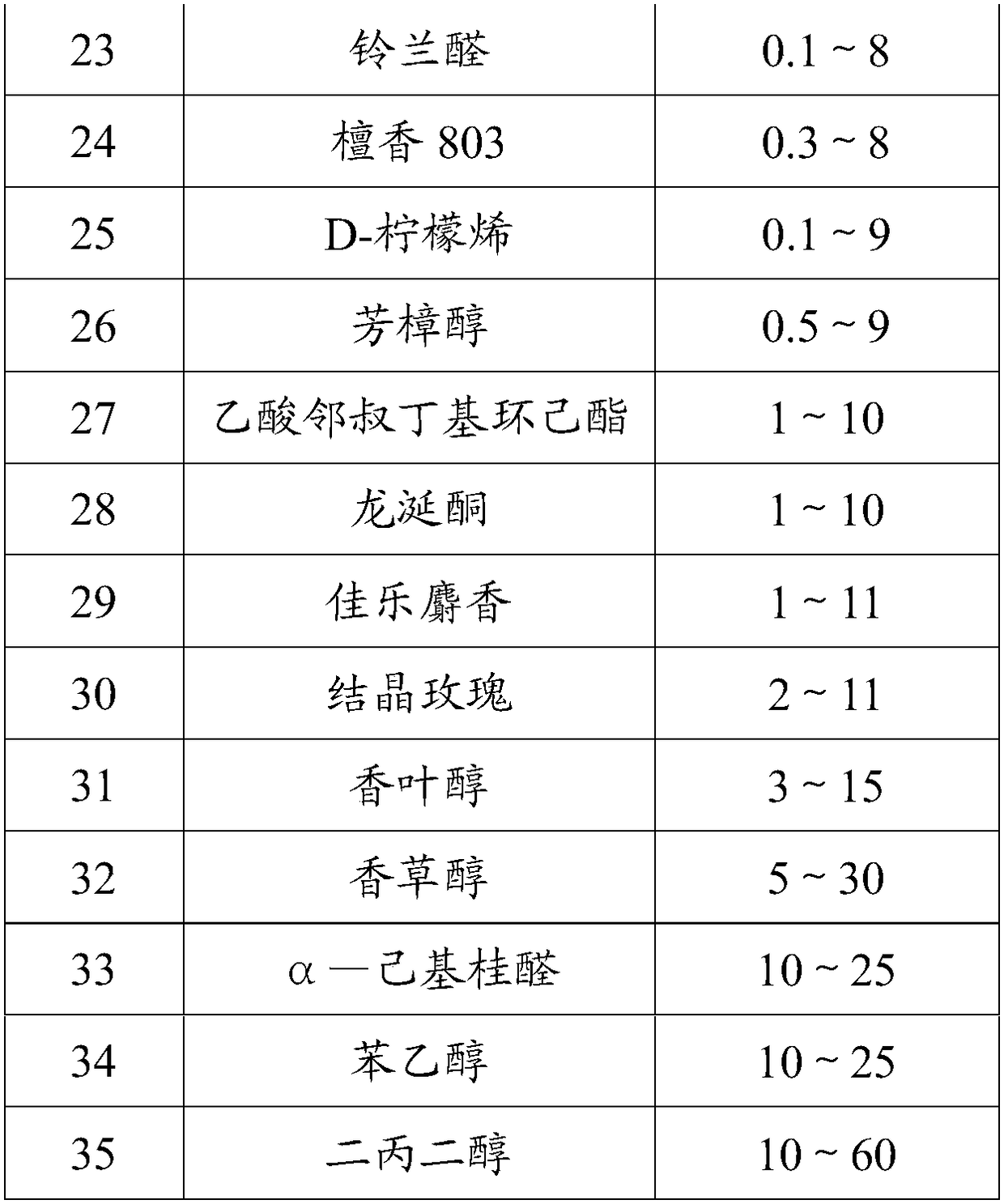
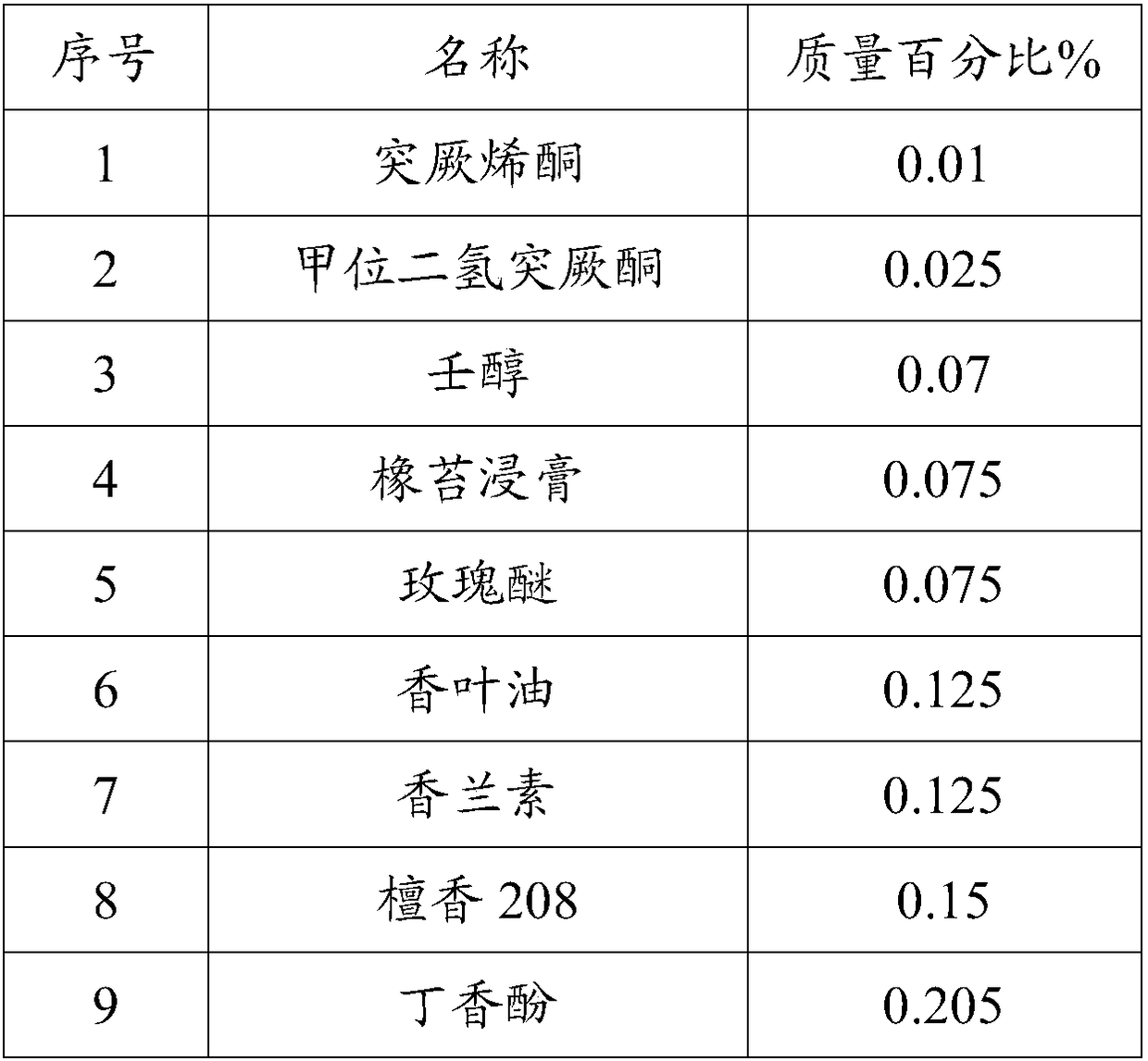
High-temperature resistance rose perfume and preparation method thereof
InactiveCN108219944AHigh degree of simulationImprove high temperature resistanceEssential-oils/perfumesEthyl phenylacetateGalaxolide
The invention discloses high-temperature resistance rose perfume and a preparation method thereof. The high-temperature resistance rose perfume is prepared from the following components according to apreset ratio: damascenone, damascone alpha, rose oxide, nonanol, geranium oil, vanillin, eugenol, sandal 208, benzyl acetate, ethyl phenylacetate, rosone, cinnamic alcohol, nerol, methyl cedryl ketone, 4-tert-butyl cyclohexyl acetate, geranyl acetate, benzyl benzoate, sandal 803, o-tert-butyl cyclohexyl acetate, geraniol, vanillin alcohol, phenylethyl alcohol, dipropylene glycol, an oak moss extractum, herba pogostemonis oil, tonalid, peach aldehyde, iso-mythyl ionone, bangalol radjanol, lilial, D-limonene, linalool, ambrotone, galaxolide and alpha-hexyl cinnamal. The rose perfume provided bythe invention is high in simulation degree and high temperature resistance ability.
Owner:鼎和(东莞)生物科技有限公司
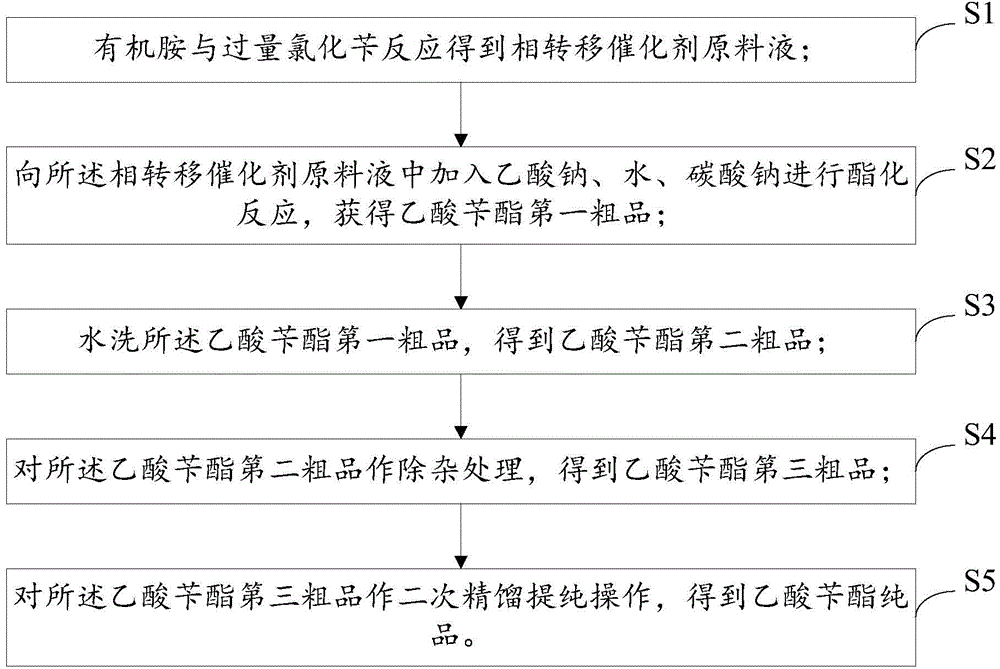
Preparation method of benzyl acetate
InactiveCN104402719AReduce complexityLow costOrganic compound preparationCarboxylic acid esters preparationSodium acetateBenzyl acetate
The invention discloses a preparation method of benzyl acetate. The method comprises the following steps: enabling organic amine to react with excessive benzyl chloride at the temperature of 60-90 DEG C for 15-60 min so as to obtain a phase transfer catalyst feed solution; adding sodium acetate, water and sodium carbonate into the phase transfer catalyst feed solution for performing esterification reaction so as to obtain a first crude product of the benzyl acetate; washing the first crude product of the benzyl acetate with water so as to obtain a second crude product of the benzyl acetate; performing impurity removal treatment on the second crude product of the benzyl acetate so as to obtain a third crude product of the benzyl acetate; performing secondary rectification and refining operation on the third crude product of the benzyl acetate so as to obtain the pure product of the benzyl acetate. According to the invention, a homemade phase transfer catalyst is used for reaction, so that the cost is reduced, the complexity of a process route is greatly reduced, the by-product, that is benzyl alcohol, is translated into the benzyl acetate product, and the reaction yield and the conversion rate are effectively increased. The characteristics of high yield, good purity, and pure fragrance of the product are achieved.
Owner:FUZHOU FUDA HUIXIANG CHEM TECH
Preparation method of cigarette essence with jasmine flower smelling fragrance and application
InactiveCN106381224AUnique and Elegant SmellTo meet the needs of novel and unique aspectsTobacco preparationEssential-oils/perfumesBiotechnologyIsoeugenol
The invention provides a preparation method of cigarette essence with jasmine flower smelling fragrance and application. The essence is prepared from the following raw materials of benzyl alcohol, phenethyl alcohol, jasmine clear oil, benzyl acetate, beaver clear oil, isoeugenol, cedrol, geraniol, cinnamyl alcohol, diethyl phthalate, ethyl alcohol and propanediol; the materials are stirred and mixed and are subjected to still standing. The jasmine flower essence is applied into filter tip connecting glue, so that the smelling fragrance of the cigarette is obvious; in addition, airtight filter rod forming paper is used for blocking the entering of mainstream smoke gas during the smoking. The cigarette product produced by using the method has the obvious jasmine flower smelling fragrance; in addition, the fragrance harmonizes with the tobacco fragrance. The application method is particularly suitable for the preparation of the smelling fragrance flavor cigarette with poor harmony with the tobacco essence.
Owner:HUBEI CHINA TOBACCO IND
Banana essence
The invention discloses a banana essence. The banana essence is characterized by being prepared from, by weight, 0.2-0.4% of vanillin, 8-10% of isoamyl acetate, 0.05-0.08% of linalyl acetate, 0.06-0.08% of linalool, 0.1-0.25% of benzyl acetate, 1.1-1.3% of ethyl butyrate, 2-2.5% of isoamyl butyrate, 0.3-0.4% of ethyl caproate, 0.05-0.1% of eugenol, 0.01-0.03% of citral, 5-7% of sweet orange base, 0.002-0.008% of furanone, 60-65% of ethyl alcohol and the balance distilled water. The banana essence has the advantages of being simple in formula, pure in fragrance, low in cost, especially suitable for being applied to frozen food, and capable of bringing a strong summer freshness sense to people.
Owner:SHANGHAI PEACOCK FLAVORS & FRAGRANCES TECH CO LTD
Green apple essence used for water-based ink and preparation method of green apple essence
InactiveCN105132169ANo stratificationSystem stabilityEssential-oils/perfumesPropanoic acidGalaxolide
The invention provides green apple essence used for water-based ink. The green apple essence is prepared from, by weight, 1-30% of green apple flavor, 5-55% of composite modified oligosaccharide and deionized water. The green apple flavor is prepared from ligustral, leaf alcohol, cis-3-hexenyl acetate, cis-3-hexenyl isovalerate, trans-2-hexenal, trans-2-hexenyl acetate, styralyl propionate, linalool, linalyl acetate, fructone, peach aldehyde, isoamyl isovalerate, isoamyl acetate, allyl caproate, sweet orange oil, acetic acid, acetic acid, geranyl acetate, damascene, benzyl acetate, lilial, cedryl acetate, acetoxy-2-tert-butylcyclohexane, ethyl acetoacetate, tonkalide, galaxolide and ethyl alcohol. Composite modified oligosaccharide serves as a wall material for wrapping green apple essence, green apple nanometer capsule slow-release essence is formed, and therefore the fragrance holding property and the water solubility of the essence are improved, and the green apple essence is suitable for perfuming of water-base ink.
Owner:SHANGHAI INSTITUTE OF TECHNOLOGY
Nanometre complex solid superacid and preparation and application thereof
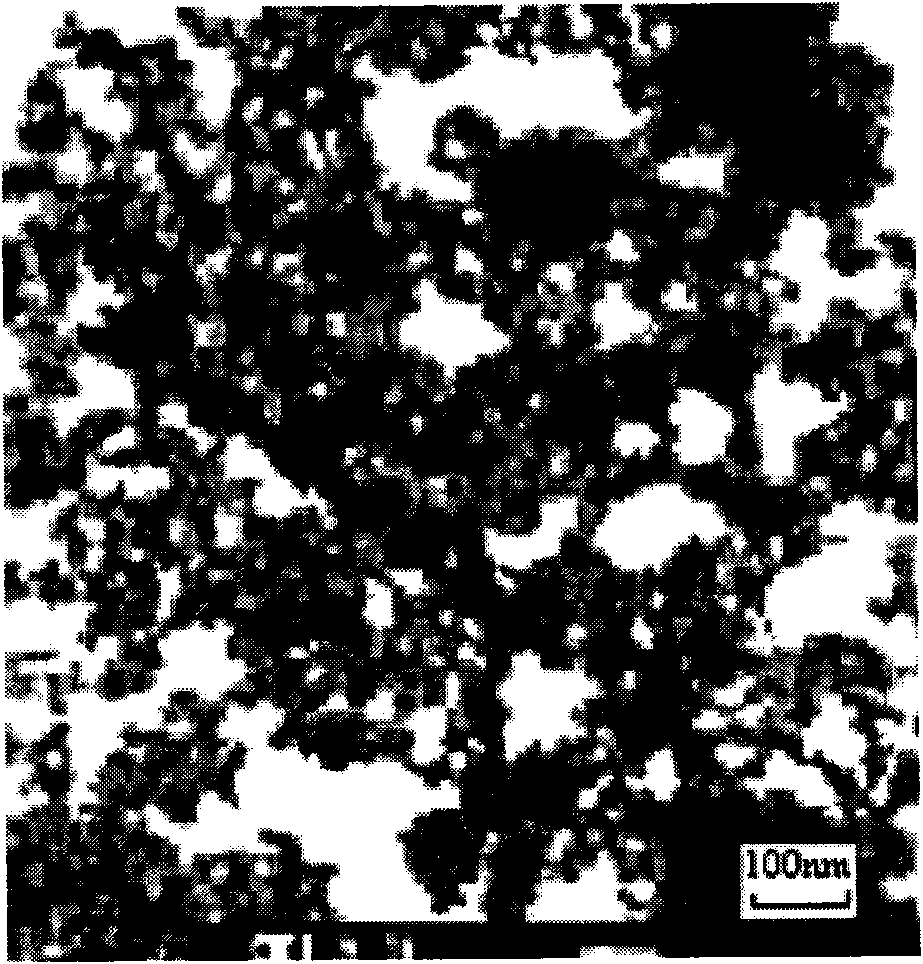
InactiveCN101596458ALarge specific surface areaHigh acid catalytic activityOrganic compound preparationCarboxylic acid esters preparationSulfurBenzyl acetate
The invention discloses a nanometre complex solid superacid which is a composite S2O8 / ZnFe2O4 composed of S2O8 and ZnFe2O4. The nanometre complex solid superacid is in spinel structure, the average grain diameter of crystal grain is 20-60nm, acid strength is -11.99--14.52, specific surface area is 120-190m / g, and the mass percentage of sulphur is 1.8-6.5%. The catalyst is prepared by using a coprecipitation-immersion method with simple preparation route and low cost, and the obtained catalyst has the advantages of complete crystal form, small crystal particle diameter, big specific surface area, high catalytic activity, long service life and the like. When used for catalyzing and synthesizing benzyl acetate, the nanometre complex solid superacid S2O8 / ZnFe2O4 of the invention has the esterification rate of 98.0%, and has the advantages of simple and easily-controlled operation, no three-waste pollution, good product quality and low production cost.
Owner:ANKANG UNIV
Preparation of benzyl acetate
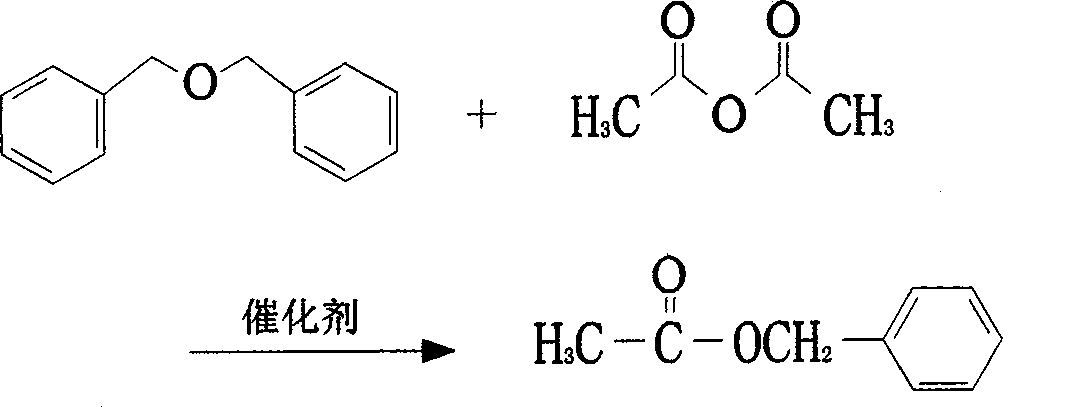
ActiveCN101434539AEasy to operateThree wastes less pollutionOrganic compound preparationCarboxylic acid esters preparationAcetic anhydrideAlcohol
The invention discloses a method for producing jasmal, which mainly adopts dibenzyl ether which is a high boiling waste produced by benzoic alcohol, as a raw material. The dibenzyl ether reacts with acetic anhydride in the presence of solid acid catalysts to obtain a crude product, and then the crude product is refined and purified to obtain an end product. The technology has simple operation process, stability, high efficiency, less pollution of the three wastes and applicability to the industrialized production.
Owner:WUHAN YOUJI IND
Washing essence comprising 2-pentylcyclopentanone
Washing essence comprising 2-pentylcyclopentanone is disclosed. The washing essence comprises following components by mass: 0.1% of ethyl 2-methylbutyrate, 0.1% of ethyl 2-methylvalerate, 0.2% of octyl aldehyde, 0.6% of D-limonene, 0.7% of p-methyl anisole, 1% of dihydromyrcenol, 0.4% of allyl hexanoate, 2% of triplal, 2% of linalool, 0.6% of methyl benzoate, 0.1% of rose oxide, 4% of phenethyl alcohol, 0.2% of camphor, 4% of benzyl acetate, 1.5% of styralyl acetate, 0.4% of terpilenol, 0.7% of decanal, 2% of citronellol, 0.2% of allyl amyl glycolate, 0.1% of citral, 0.5% of geraniol, 0.1% of linalyl acetate, 0.8% of anisic aldehyde, 3% of the 2-pentylcyclopentanone, 1.2% of dipropylene glycol, and the like. Using of the 2-pentylcyclopentanone allows the whole fragrance of the essence to be finer and fuller, and aroma reserving effects to be more durable.
Owner:TIANJIN DOUBLE HORSE FLAVOR & FRAGRANCE NEW TECH
Jasmine essence
InactiveCN101760311ARich fragranceStrong persistenceEssential-oils/perfumesJasminum grandiflorumEugenol
The invention discloses a jasmine essence, which is characterized in that: the jasmine essence comprises the following components in percentage by weight: 30.0 to 35.0 percent of benzyl acetate, 0.8 to 1.0 percent of benzyl benzoate, 7.0 to 7.5 percent of linalool, 1.0 to 1.2 percent of alpha-methyl ionone, 7.0 to 7.5 percent of bergamio, 0.4 to 0.6 percent of alpha-terpineol, 5.0 to 7.0 percent of phenethyl alcohol, 4.0 to 5.0 percent of Jasminum grandiflorum absolute, 15.0 to 17.0 percent of benzyl alcohol, 0.4 to 0.6 percent of eugenol, 5.0 to 5.5 percent of hydroxycitronellal, 4.0 to 4.5 percent of ylang ylang oil, 5.0 to 6.0 percent of amyl cinnamaldehyde diethylacetal, 0.8 to 1.1 percent of aglaia oil, 1.6 to 2.0 percent of narcelo, 0.3 to 0.5 percent of indole and 1.8 to 2.0 percent of alpha-amyl cinnamaldehyde schiff's base. The jasmine essence with the formula is suitable for fragrant products such as white cream, lotion, fragrant molasses and the like, does not have the defect of color change, and has the advantages of pure and rich fragrance, strong persistance, low consumption and the like.
Owner:张彬
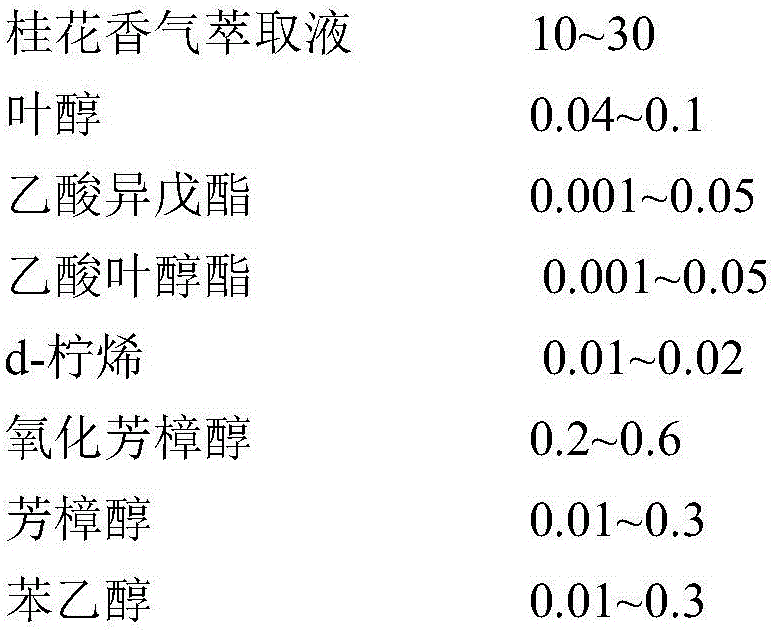
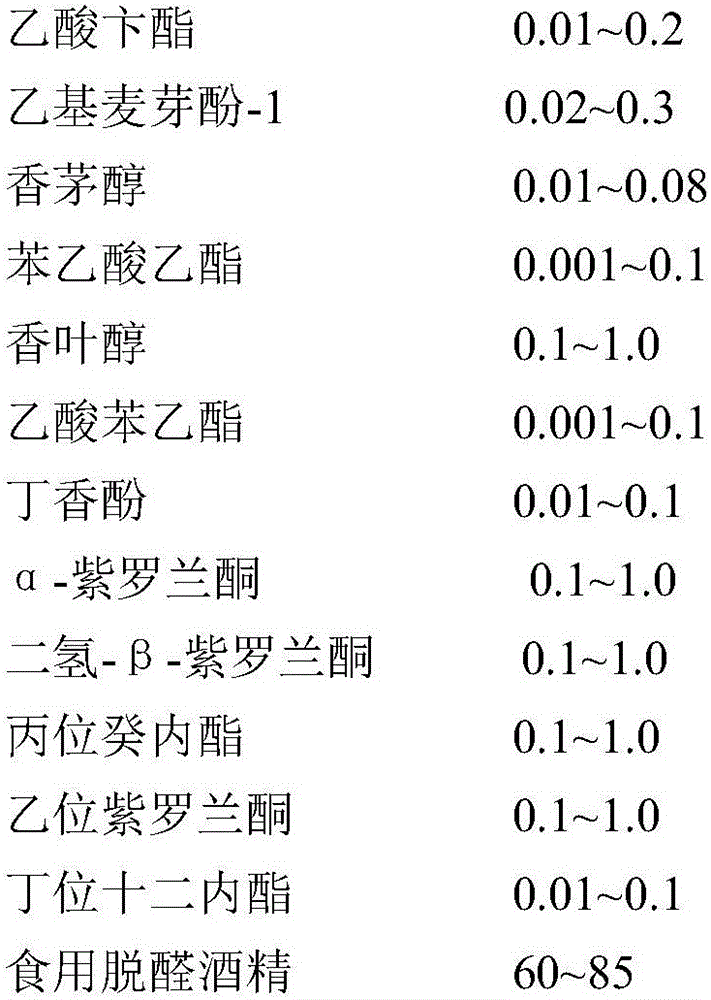
Osmanthus flower essence containing natural osmanthus flower fragrance and preparation method thereof
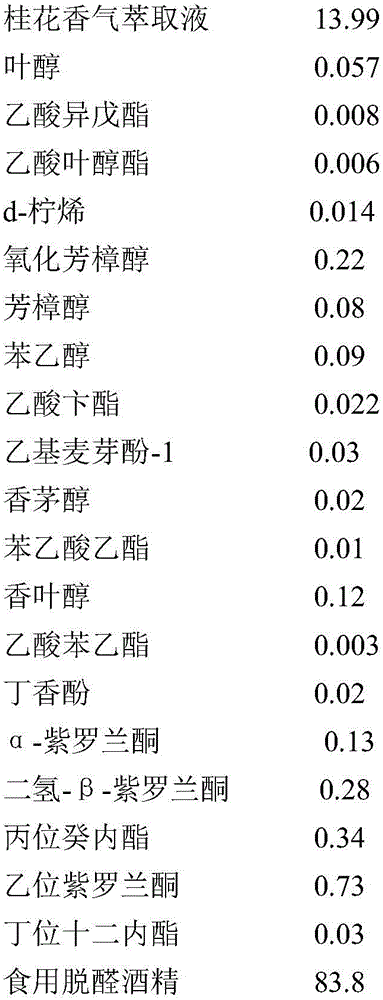
InactiveCN106367204AIncrease aromaGreat tasteEssential-oils/perfumesFood ingredient as flavour affecting agentFood additiveEthyl phenylacetate
The invention discloses an osmanthus flower essence containing natural osmanthus flower fragrance and a preparation method thereof. The osmanthus flower essence mainly comprises natural osmanthus flower fragrance extract, leaf alcohol, isoamyl acetate, cis-3-hexenyl acetate, d-limonene, linalool oxide, linalool, phenethyl alcohol, benzyl acetate, ethyl maltol-1, citronellol, ethyl phenylacetate, geraniol, phenethyl acetate, eugenol, alpha-ionone, dihydro-beta-ionone, gamma-decalactone, beta-ionone, delta-dodecalactone, edible alcohol and like components. The above preparation has the following advantages: the osmanthus flower fragrance extract obtained by low-temperature vacuum distillation serves as a key raw material, and the osmanthus flower essence is prepared by means of an analog-matching formulation. The osmanthus flower essence is featured with verisimilar fragrance, rich body fragrance, strong natural feeling and lasting base fragrance, is applied to food and beverage as food additive, and can bring product natural osmanthus flower fragrance and mouth feel; and meanwhile the fragrance quality and fragrance amount of the osmanthus flower essence are higher than like essence products at home.
Owner:HANGZHOU EVER MAPLE FLAVOR & FRAGRANCE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com