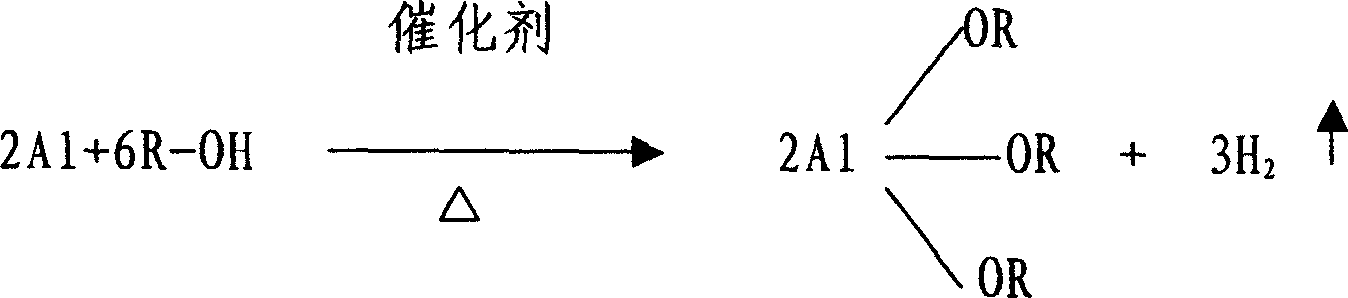
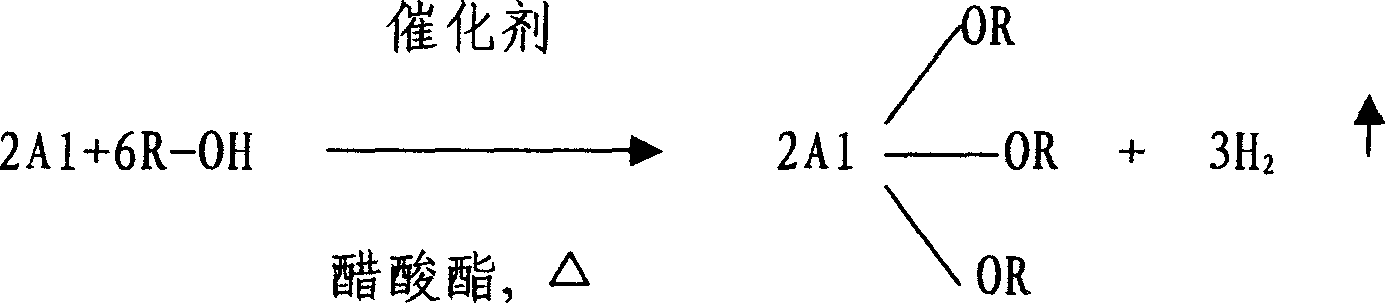Method for fabricating aluminum alkoxide
A manufacturing method and technology for aluminum alkoxide, applied in the field of manufacturing aluminum alkoxide, can solve the problems of increased manufacturing cost of aluminum alkoxide, denaturation of aluminum alkoxide mixture, unsafe production of aluminum alkoxide, etc., so as to improve the conversion rate, reduce the addition amount, and reduce impurities. The effect of content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] In a 3-liter three-necked flask with a reflux condenser, put 150g of aluminum particles with a purity of 99%, 0.5g of anhydrous aluminum trichloride, and 1kg of methyl acetate solvent, then add 0.5kg of isopropanol, stir and heat. Control the temperature of the solution at about 60°C for reflux reaction. After 2 hours, a black aluminum isopropoxide mixture solution is obtained, and the material is discharged by suction. After 24 hours of settlement, the upper layer is sucked out of a colorless and transparent solution, which contains aluminum isopropoxide 36%, heating to 60°C to start distillation, with the evaporation of methyl acetate, the distillation temperature is gradually increased, when it reaches 80°C, stop heating, and after cooling, the finished product of aluminum isopropoxide is obtained, in which the impurity content of metal and metal oxide is 0.01% the following. The evaporated methyl acetate can be recycled.
Embodiment 2
[0020] In a 3-liter three-neck flask with a reflux condenser, put 150g of aluminum particles with a purity of 99%, 0.5g of anhydrous aluminum trichloride, 1.2kg of ethyl acetate solvent, and then add 0.4kg of isopropanol, stir and heat , control the temperature of the solution at about 80°C for reflux reaction. After 2 hours, a black aluminum isopropoxide mixture solution is obtained, and the material is discharged by suction. After 30 hours of settlement, the upper layer is sucked out of a colorless and transparent solution, which contains isopropanol Aluminum 2 7%, heating to 80 ° C to start distillation, with the evaporation of ethyl acetate, the distillation temperature is gradually increased, when it reaches 97 ° C, stop heating, and after cooling, the finished product of aluminum isopropoxide is obtained, in which the content of metal and metal oxide impurities is 0.01% or less. The evaporated ethyl acetate can be recycled.
Embodiment 3
[0022] In a 3-liter three-neck flask with a reflux condenser, put 150g of aluminum particles with a purity of 99%, 0.14g of anhydrous aluminum trichloride, 1kg of isopropyl acetate solvent, and then add 0.4kg of isopropanol, stir and heat , control the temperature of the solution at about 90°C for reflux reaction, after 2 hours, a black aluminum isopropoxide mixture solution is obtained, the material is pumped out, and after 15 hours of settlement, the upper layer is sucked out of a colorless and transparent solution, which contains isopropanol Aluminum 30%, heating to 90 ° C to start distillation, with the evaporation of isopropyl acetate, the distillation temperature is gradually increased, when it reaches 110 ° C, stop heating, and after cooling, the finished product of aluminum isopropoxide is obtained, in which the content of metal and metal oxide impurities is 0.01% or less. The evaporated isopropyl acetate can be recycled.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com