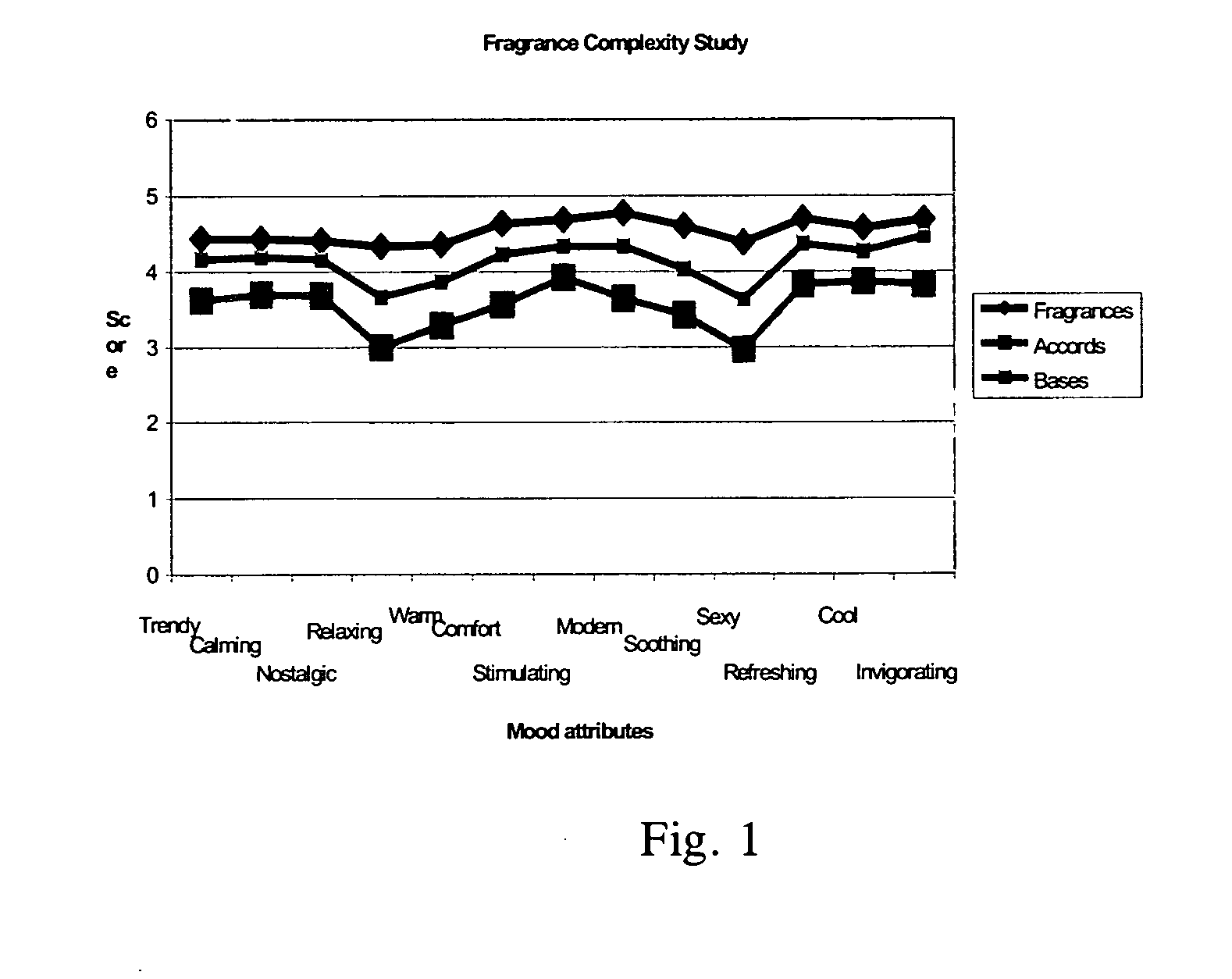
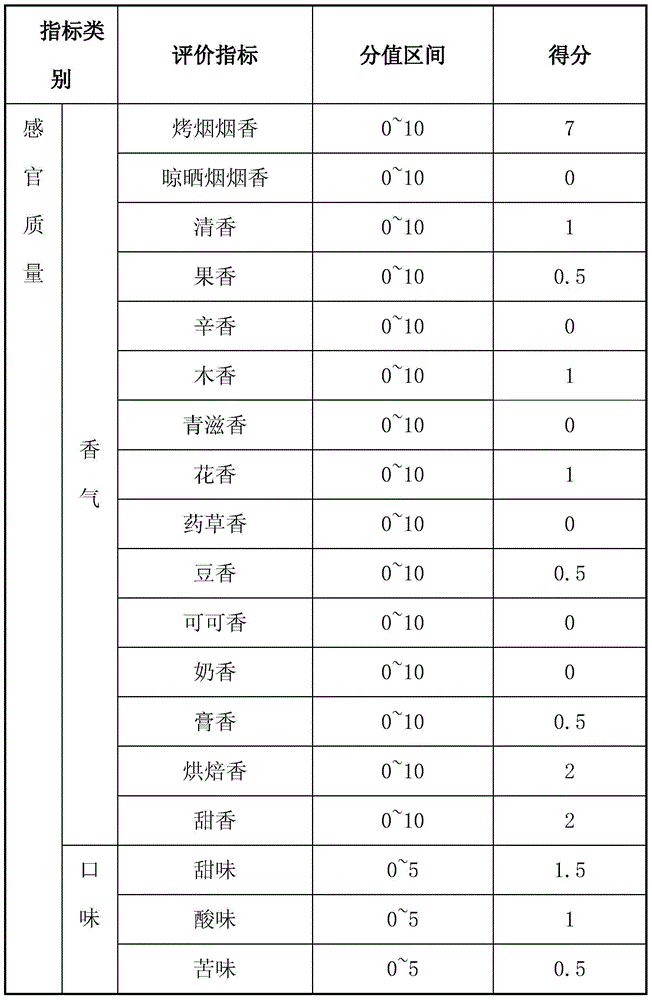
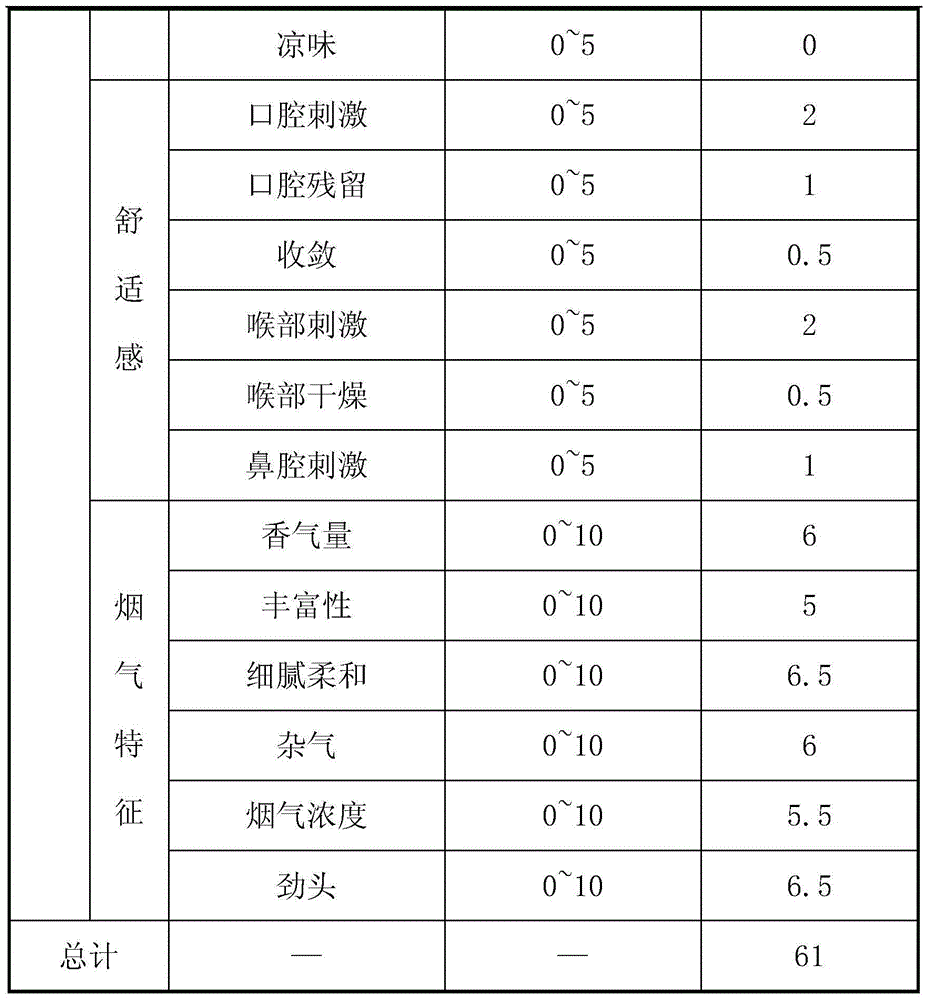
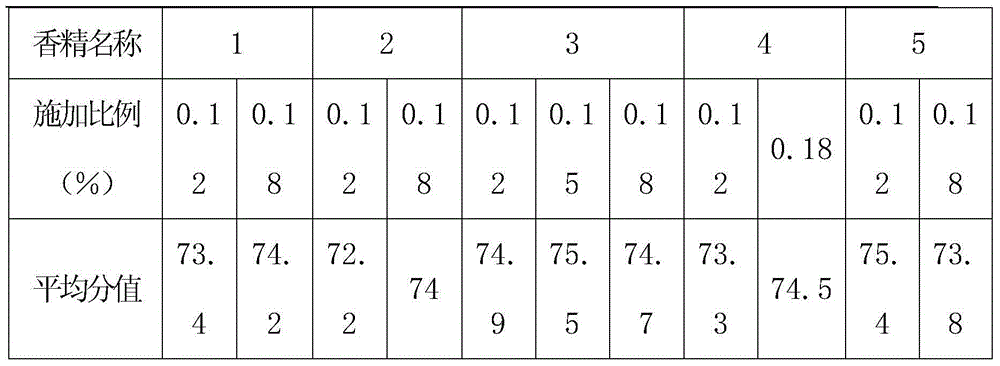
Patents
Literature
59 results about "Hydroxycitronellal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
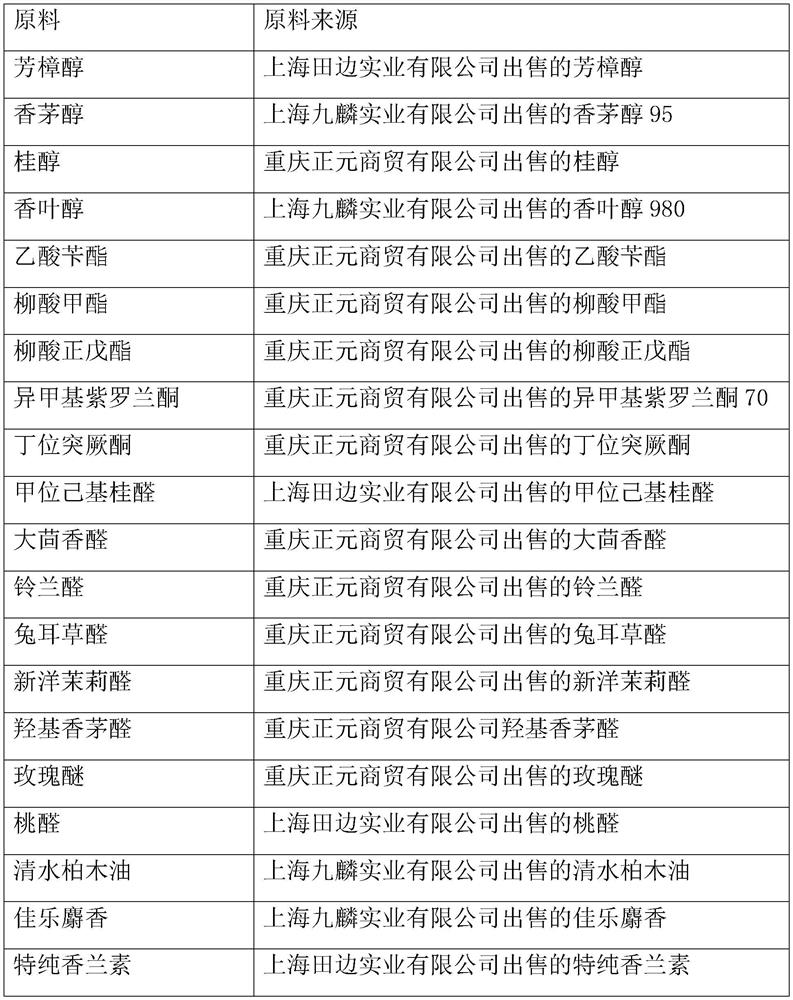
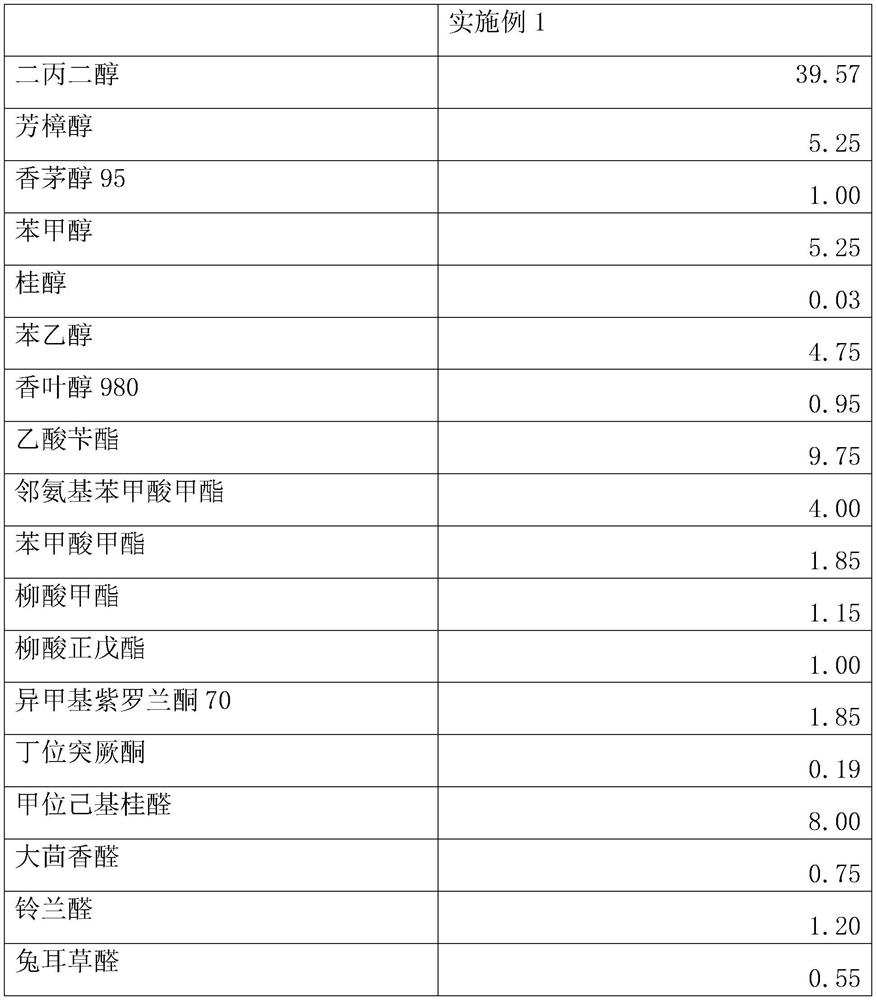
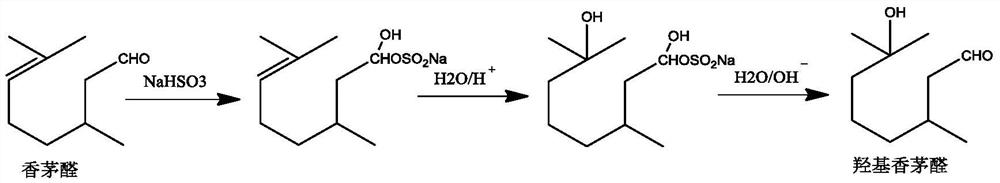
Hydroxycitronellal (7-hydroxy-3,7-dimethyloctanal) is an odorant used in perfumery. Its molecular formula is C₁₀H₂₀O₂.
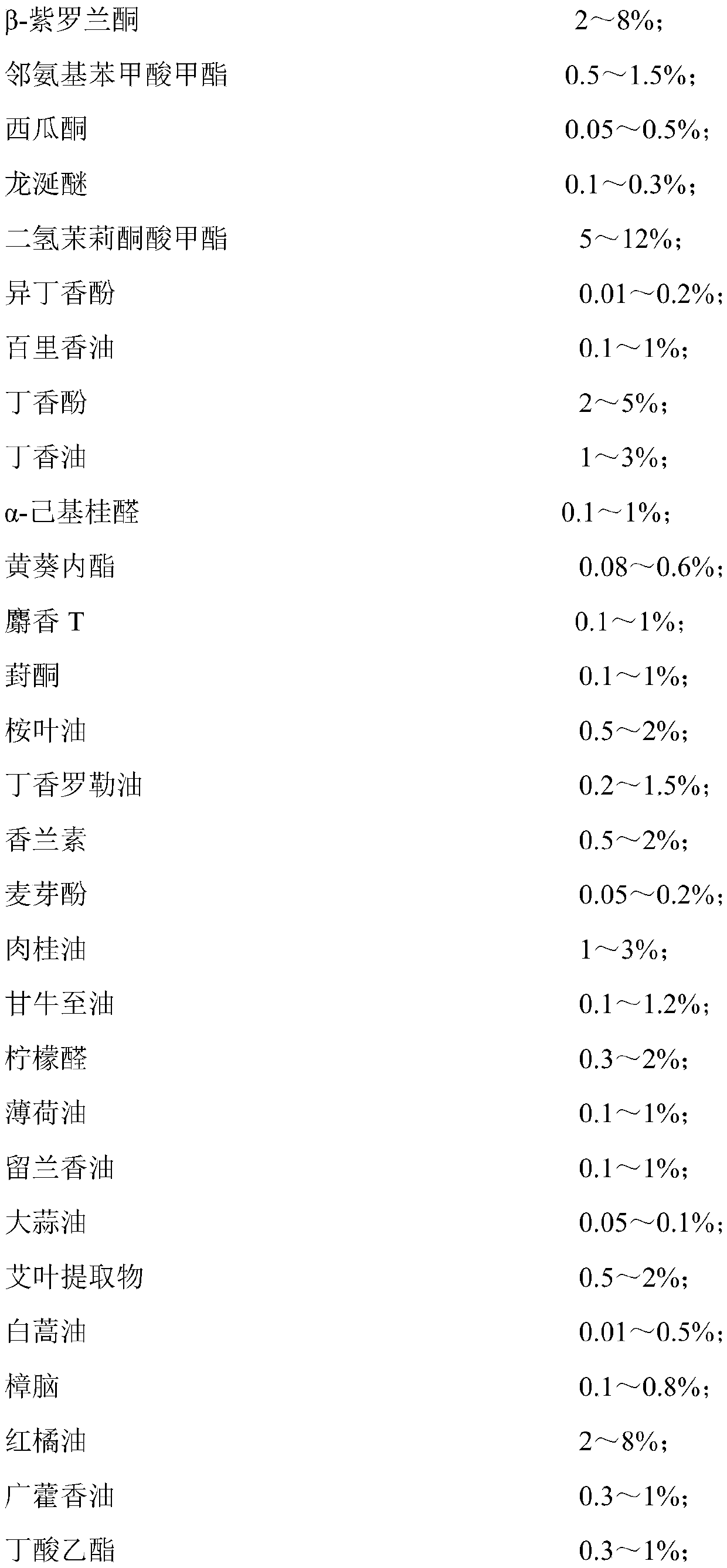
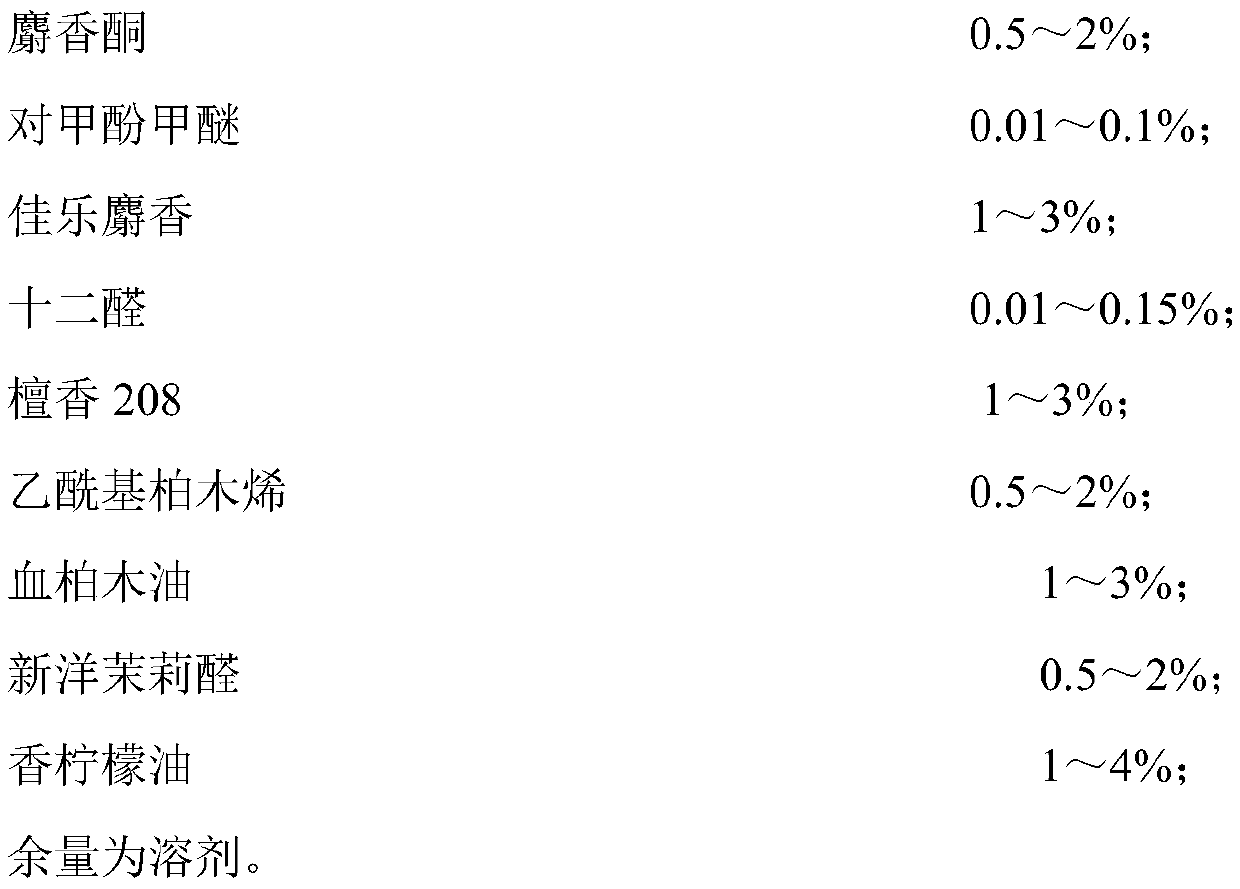
Fragrance compositions
InactiveUS20080096790A1Minimally disruptiveEasy to moveCosmetic preparationsToilet preparationsHexyl acetateLemon oil
A method of promoting activated, pleasant moods through the inhalation of energising, non-stressing fragrances (invigorating fragrances) comprising at least 75% by weight, preferably 85% by weight of perfume materials drawn from the following groups:A) At least 10% by weight in total of at least three materials drawn from Group ‘IMP’ comprising: allyl amyl glycolate; benzyl salicylate; bergamot oil; coriander oil; cyclamen aldehyde; 1-(2,6,10-trimethylcyclododeca-2,5,9-trien-1-yl)ethanone; allyl (cyclohexyloxy)acetate; Damascenia 185 SAE; 2,4-dimethylheptan-1-ol; fir balsam; fir needle oil; 3-(4-ethylphenyl)-2,2-dimethylpropanal; ginger oil; guaiacwood; linalyl acetate; litsea cubeba oil; methyl 2,4-dihydroxy-3,6-dimethylbenzoate; nutmeg oil; olibanum oil; orange flower oil; Ozonal AB 7203C; patchouli oil; rose oxide; rosemary oil; sage clary oil; spearmint oil; Tamarine AB 8212E; tarragon oil;B) Optionally up to 90% of materials from the following groups:Group ‘HMR’ comprising:allyl ionone; benzyl acetate; cis-jasmone; citronellol; ethyl linalol; ethylene brassylate; 4-methyl-2-(2-methylpropyl)tetrahydro-2H-pyran-4-ol; geraniol; geranium oil; isoeugenol; lemon oil; 2,4-dimethylcyclohex-3-ene-1-carbaldehyde; 3-(4-hydroxy-4-methylpentyl)cyclohex-3-ene-1-carbaldehyde; 4-(4-hydroxy-4-methylpentyl)cyclohex-3-ene-1-carbaldehyde; alpha-iso-methyl ionone; 3-methylcyclopentadec-2-en-1-one; cyclopentadecanone; cyclohexadecanolide; gamma-undecalactone.Group ‘HMI’ comprising:1-{[2-(1,1 -dimethylethyl)cyclohexyl]oxy}butan-2-ol; 3a,6,6,9a-tetramethyldodecahydronaphtho[2,1 -{b}]furan; alpha-damascone; dihydromyrcenol; eugenol; 3-(1,3-benzodioxol-5-yl)-2-methylpropanal; 2,4-dimethylcyclohex-3-ene-1-carbaldehyde; mandarin oil; orange oil; 2-(1,1-dimethylethyl)cyclohexyl acetate.Group ‘HMP’ comprising:1-(2,6,6,8-tetramethyltricyclo[5.3.1.0 {1,5}]undec-8-en-9-yl)ethanone; allyl cyclohexyl propionate; allyl heptanoate; Apple Oliffac S pcmf; 7-methyl-2H-1,5-benzodioxepin-3(4H)-one; cassis base; cis-3-hexenyl salicylate; damascenone; gamma-decalactone; ethyl acetoacetate; ethyl maltol; ethyl methyl phenylglycidate; hexyl acetate; (3E)-4-methyldec-3-en-5-ol; 2,5,5-trimethyl-6,6-bis(methyloxy)hex-2-ene; 4-(4-hydroxyphenyl)butan-2-one; styrallyl acetate; 2,2,5-trimethyl-5-pentylcyclopentanone; ylang oil. Group ‘RMP’ comprising: anisic aldehyde; (2Z)-2-ethyl-4-(2,2,3-trimethylcyclopent-3-en-1-yl)but-2-en-1-ol; benzoin siam resinoid; ethyl vanillin; oxacyclohexadec-12(13)-en-2-one; hexyl salicylate; hydroxycitronellal; jasmin oil; 3-methyl-5-phenylpentan-1-ol; 2-(phenyloxy)ethyl 2-methylpropanoate; alpha-terpineol; vanillin;Group ‘GEN’ comprising:cyclopentadecanolide; oxacyclohexadecan-2-one; hexyl cinnamic aldehyde; ionone beta; isobornyl cyclohexanol; 1-(2,3,8,8-tetramethyl-1,2,3,4,5,6,7(8),8(8a)-octahydronaphthalen-2-yl)ethanone; 4-(1,1-dimethylethyl)phenyl]-2-methylpropanal; linalol; methyl dihydrojasmonate; 2-phenylethanol;provided the following conditions are met:(a) IMPs>=HMPs+HMRs(b) IMPs+HMIs+GENs>=70%(c) (IMP+HMI) / (IMP+HMI+RMP+HMR)>=0.7(d) IMPs / (HMPs+RMPs+IMPs)>=0.5(e) IMPs / [(HMPs+RMPs+IMPs)+(100−TOTAL)]>=0.3wherein ‘IMPs’ indicates the sum of the percentages of materials within Group IMP, and similarly for the remaining groups, the symbol ‘>=’ indicates ‘at least equal to’, and ‘TOTAL’ is the sum of HMPs, HMRs, HMIs, IMPs, RMPs and GENs, provided also that low odour or no odour solvents are excluded from the calculation of these sums is provided which have an invigorating effect when inhaled by a subject.
Owner:GIVAUDAN NEDERLAND SERVICES
Flavored essence for increasing cigarette sour and sweet note
ActiveCN104886761AIncrease sweetnessEasy extractionTobacco treatmentDihydroactinidiolideFlavoring essences
The invention relates to an essence, in particular to a flavored essence for increasing cigarette sour and sweet note. The flavored essence is made of raw materials including, in weight percent, 0.5-0.8% of 1% hydroxycitronellal, 1-2% of ethyl acetate, 5-8% of isoamyl acetate, 0.01-0.5% of beta-damascenone, 0.01-0.8% of dihydroactinidiolide, 0.01-0.8% of alpha-ionone, 1-2.5% of vanillin, 0.5-4% of butyric acid butylester, 0.5-5% of acetic acid, 0.5-5% of lactic acid, 4-10% of dried apricot slices extract, 4-10% of plum extract, 1-5% of tomato extract, 0.5-2% of iris extract, 3-8% of Zimbabwe tobacco extract, 20-30% of propanediol and 4.1-61.67% of 70% ethyl alcohol. The flavored essence solves the problems that an existing cigarette is single in fragrance and obvious in offensive odor and stimulation, smoking satisfaction and enjoyment of the cigarette is improved, the aroma amount of the cigarette is increased, obvious sour and sweet note is given for the cigarette, the flavored essence is rich in fragrance, and stimulation is reduced.
Owner:HUBEI CHINA TOBACCO IND +1
Chrysanthemum essence for hand cream
The invention discloses a chrysanthemum essence for hand cream, which is characterized by comprising the following components by weight percent: 2 parts of vetiver oil, 2 parts of cinnamon bark oil, 14 parts of sandal oil, 28 parts of alpha-terpineol, 8 parts of jasmine fragrance base, 36 parts of geranium oil, 20 parts of ylang ylang oil, 30 parts of hydroxycitronellal, 20 parts of lavender oil, 12 parts of bergamot oil, 8 parts of isoeugenol, 2 parts of iris resin, 2 parts of musk ketone, 8 parts of Peru musk, 16 parts of heliotropin and 2 parts of vanillin. The chrysanthemum essence for the hand cream is suitable for fragrance products of white cream, skin milk, fragrant molasses and the like, has no defect of discoloration, and has the advantages of pure and rich fragrance, strong durability, extremely less using quantity and the like.
Owner:李全伟
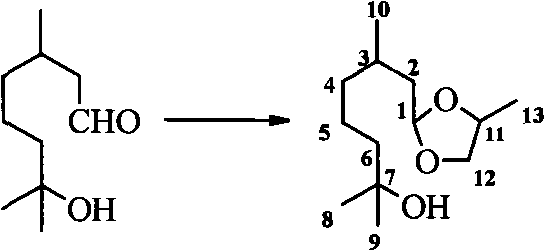
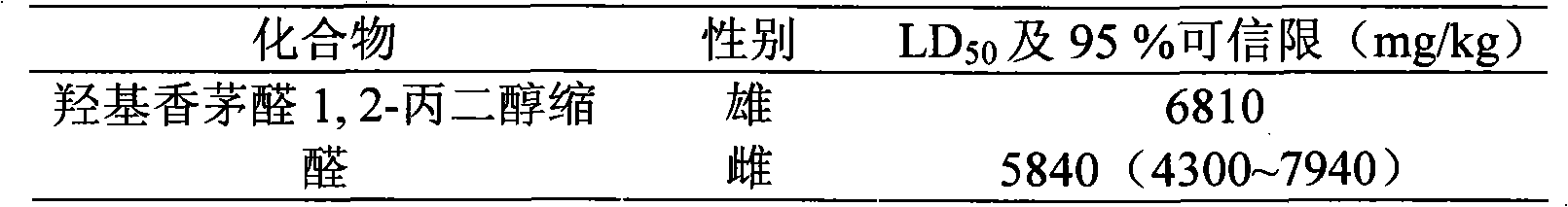
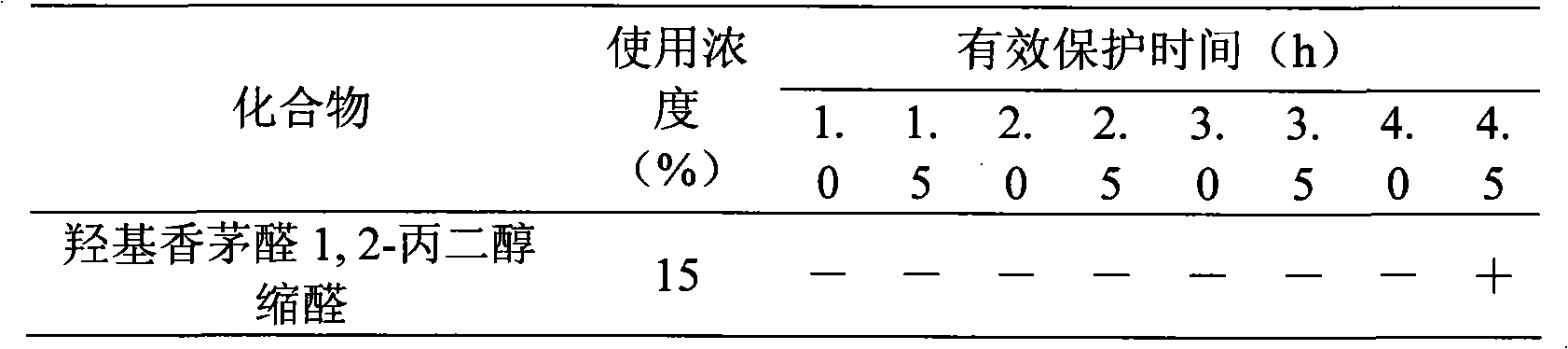
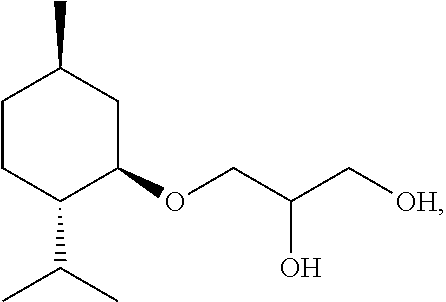
Hydroxycitronellal 1,2-propanediol acetal used as repellent
The invention relates to hydroxycitronellal 1,2-propanediol acetal used as repellent. The compound hydroxycitronellal 1,2-propanediol acetal shown as follows can be used as the insect repellent. The hydroxycitronellal 1,2-propanediol acetal of the invention can be used as the substitute of diethyltoluamide to repel Culex pipiens pallens.
Owner:JIANGXI AGRICULTURAL UNIVERSITY
Mixed type chrysanthemum essence and preparation method thereof
InactiveCN105349263AStrong aromaDelicate aromaEssential-oils/perfumesCrossostephiumPhenethyl alcohol
The invention discloses mixed type chrysanthemum essence. The mixed type chrysanthemum essence is prepared from bergamot oil, sweet orange oil, cis-3-hexenyl acetate, citral, ligustral, ethyl acetate, tetrahydrogeraniol, dimethyl benzyl orthoester, myristica oil, alpha-damascone, ethyl maltol, peach aldehyde, linalool, phenethyl alcohol, nerol, methyl dihydrojasmonate, geraniol, citronellol, hydroxycitronellal, thyme oil, crossostephium leaf extract, crossostephium leaf essence, terpilenol, caryophyllene, camphor, borneol, butyric acid, hexanal, heliotropin and propylene glycol. The invention further provides a preparation method of mixed type chrysanthemum essence. Chrysanthemum essence which is strong in fragrance, natural and vivid is obtained by conducting mixing according to the mass percent.
Owner:SHANGHAI INST OF TECH
Jasmine essence
InactiveCN101760311ARich fragranceStrong persistenceEssential-oils/perfumesJasminum grandiflorumEugenol
The invention discloses a jasmine essence, which is characterized in that: the jasmine essence comprises the following components in percentage by weight: 30.0 to 35.0 percent of benzyl acetate, 0.8 to 1.0 percent of benzyl benzoate, 7.0 to 7.5 percent of linalool, 1.0 to 1.2 percent of alpha-methyl ionone, 7.0 to 7.5 percent of bergamio, 0.4 to 0.6 percent of alpha-terpineol, 5.0 to 7.0 percent of phenethyl alcohol, 4.0 to 5.0 percent of Jasminum grandiflorum absolute, 15.0 to 17.0 percent of benzyl alcohol, 0.4 to 0.6 percent of eugenol, 5.0 to 5.5 percent of hydroxycitronellal, 4.0 to 4.5 percent of ylang ylang oil, 5.0 to 6.0 percent of amyl cinnamaldehyde diethylacetal, 0.8 to 1.1 percent of aglaia oil, 1.6 to 2.0 percent of narcelo, 0.3 to 0.5 percent of indole and 1.8 to 2.0 percent of alpha-amyl cinnamaldehyde schiff's base. The jasmine essence with the formula is suitable for fragrant products such as white cream, lotion, fragrant molasses and the like, does not have the defect of color change, and has the advantages of pure and rich fragrance, strong persistance, low consumption and the like.
Owner:张彬
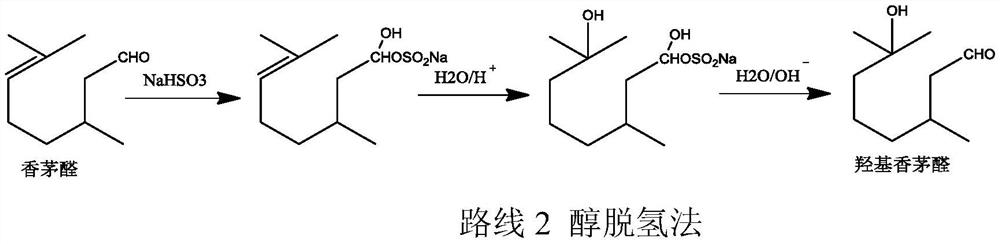
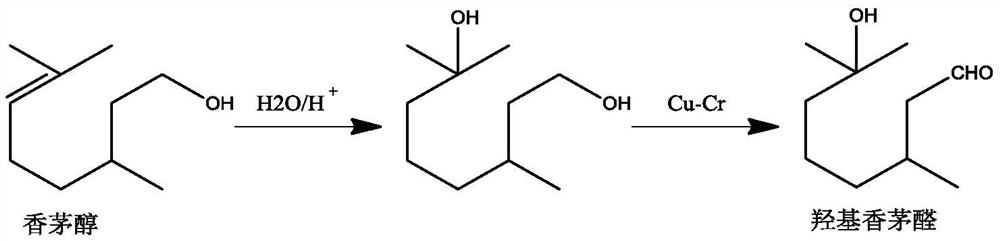
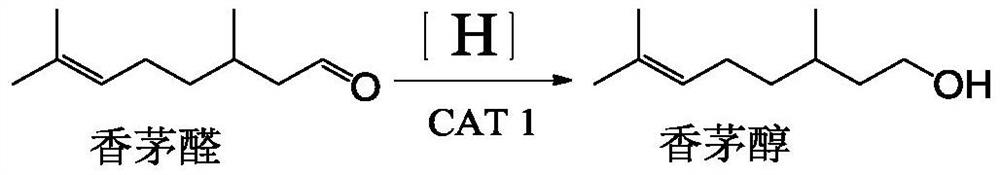
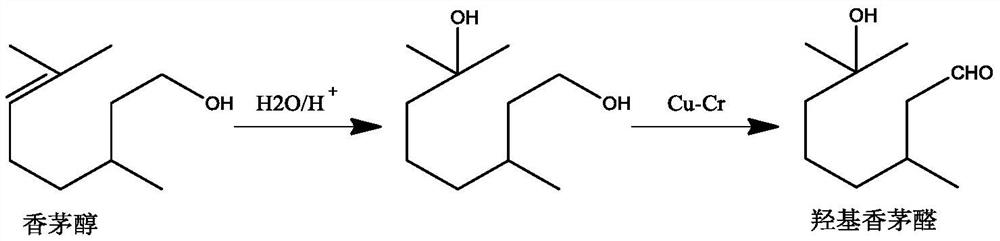
Method for preparing hydroxycitronellal through hydration reaction of citronellal
ActiveCN111825543ARich pore structureLarge specific surface areaMolecular sieve catalystsOrganic compound preparationHydration reactionMolecular sieve
The invention discloses a method for preparing hydroxycitronellal through a hydration reaction of citronellal. The method comprises the following step: under the action of a Pd-coated porous molecularsieve catalyst and an auxiliary agent, citronellal and water are subjected to a hydration reaction to synthesize hydroxyl citronellal in one step. The Pd / Si molar ratio of the Pd-coated porous molecular sieve catalyst is 1: (10-200), and the Si / Al molar ratio of the Pd-coated porous molecular sieve catalyst is (50-100): 1. The auxiliary agent is an ether substance, preferably 3-phenoxy toluene. Under the action of a porous solid catalyst and an auxiliary agent, the raw material citronellal and water are subjected to a catalytic hydration reaction to synthesize hydroxycitronellal in one step,and the method has the advantages of high selectivity, simplified process, less three wastes and the like.
Owner:WANHUA CHEM GRP CO LTD +1
Fragrance essence with antibacterial effect components and preparation method thereof
InactiveCN110129134AHas antibacterial effectAroma harmonyBiocideDead animal preservationEthyl salicylateBenzaldehyde
The invention provides a fragrance essence with bacteriostatic efficacy components and a preparation method thereof. The fragrance essence with bacteriostasis effect is prepared from acetaldehyde, hydroxycitronellal, pinene, sabinene, beta-myrcene, limonene, leaf alcohol, ligustral, benzaldehyde, linalool, Bergapten, caryophyllene, linalyl acetate, beta fenchyl alcohol, anthemene, Neryl propionate, Florol, benzyl acetate, geranyl acetate, citronellyl acetate, nerol, methyl salicylate, ethyl salicylate, ethyl salicylate, Beta Damascone, phenethyl alcohol, allyl amyl glycolate, ionone, methyl anthranilate, watermelon ketone, Ambrox, Methyl dihydrojasmonate, isoeugenol, thyme oil, eugenol and the like. The fragrant raw materials used are convenient in sources, and the obtained essence has thefragrance characteristics of fresh fragrance, flower fragrance, fruit fragrance and pungent fragrance, and also has the fresh feeling of forests and nature, the overall fragrance is fine and smooth,and the fragrance essence has bacteriostatic effect.
Owner:SHANGHAI INST OF TECH
Cat-driving essence as well as preparation method and application thereof
ActiveCN103911214AEffective in repelling catsEssential-oils/perfumesAnimal repellantsLemon oilYlang-Ylang oil
The invention discloses cat-driving essence as well as a preparation method and an application thereof. The cat-driving essence is obtained by adding a certain amount of bergamot oil, lemon oil, citral, linalyl acetate, sweet orange oil, terpilenol, terpinyl acetate, methyl ortho-aminobenzoate, decanal, geranyl acetate, lemonile, geranium oil, menthol, benzyl acetate, benzyl alcohol, linalool, dihydro jasmine with mass fraction of 10%, hydroxycitronellal, benzpyrole with mass fraction of 10%, dihydromyrcenol, methyl ionone, lyral, oil of daidai leaf, ylang ylang oil, gamma-delta-lacton, leaf alcohol, benzyl benzoate, benzyl propionate, methyl dihydrojasmonate, p-cresyl acetate, jasmonyl, phenylacetic acid, eugenol, myracaldehyde, absolute of jasmine, geraniol, cis-3-hexenyl benzoate, galaxolide with mass fraction of 50% and propylene glycol into a container in sequence, shaking uniformly, standing and ageing for two weeks. The cat-driving essence is remarkable in cat-driving effect.
Owner:福建中益制药有限公司
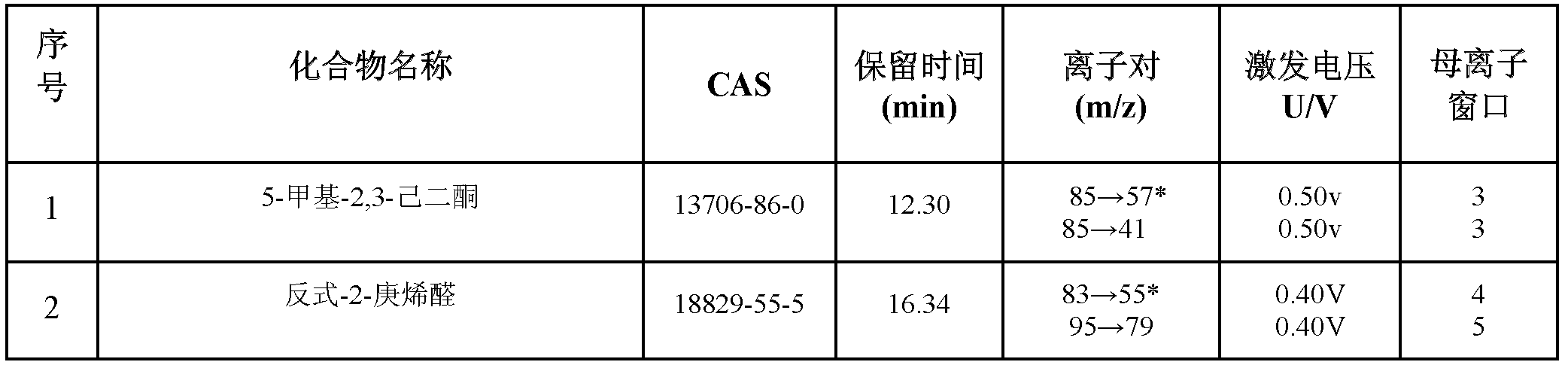
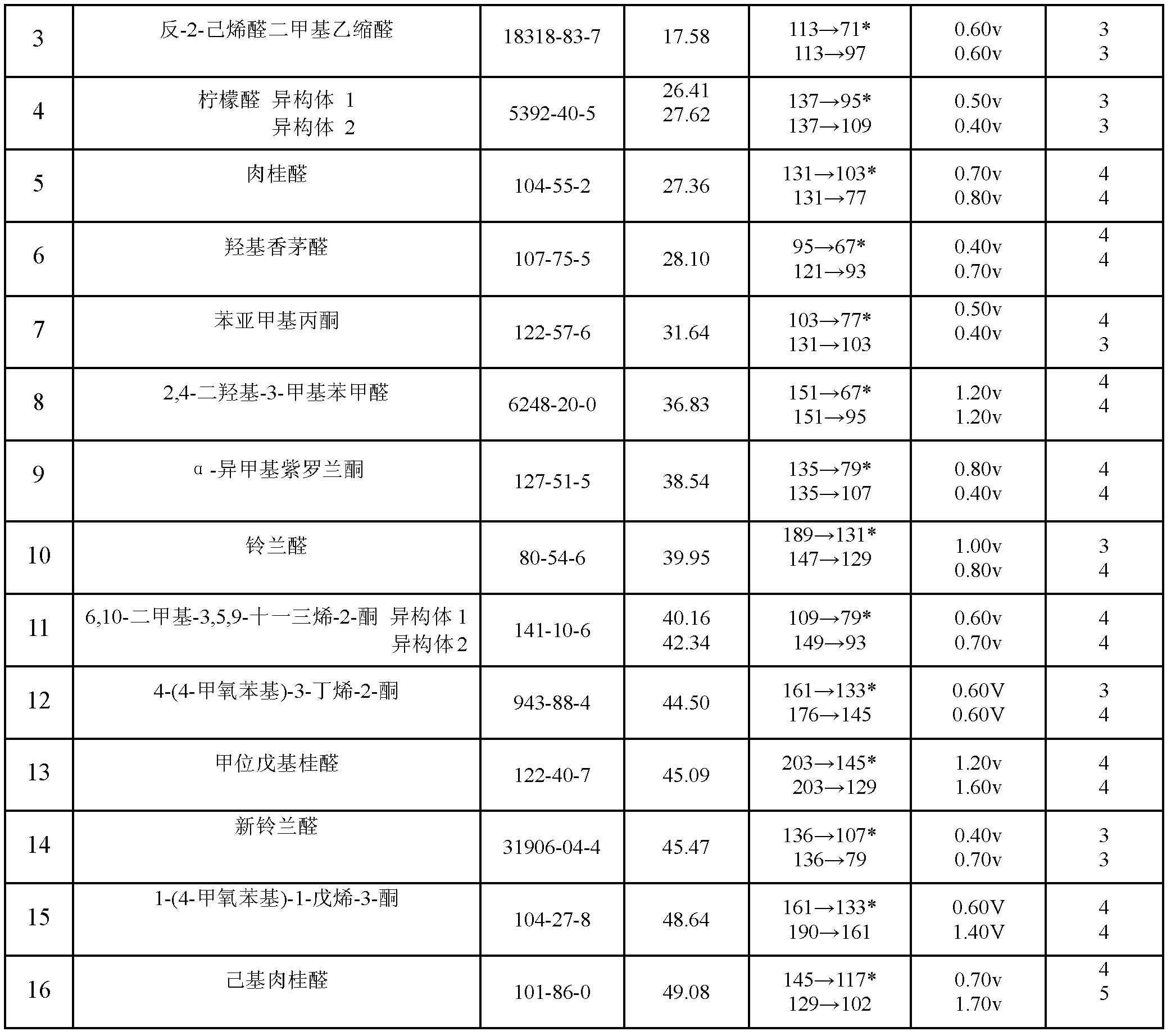
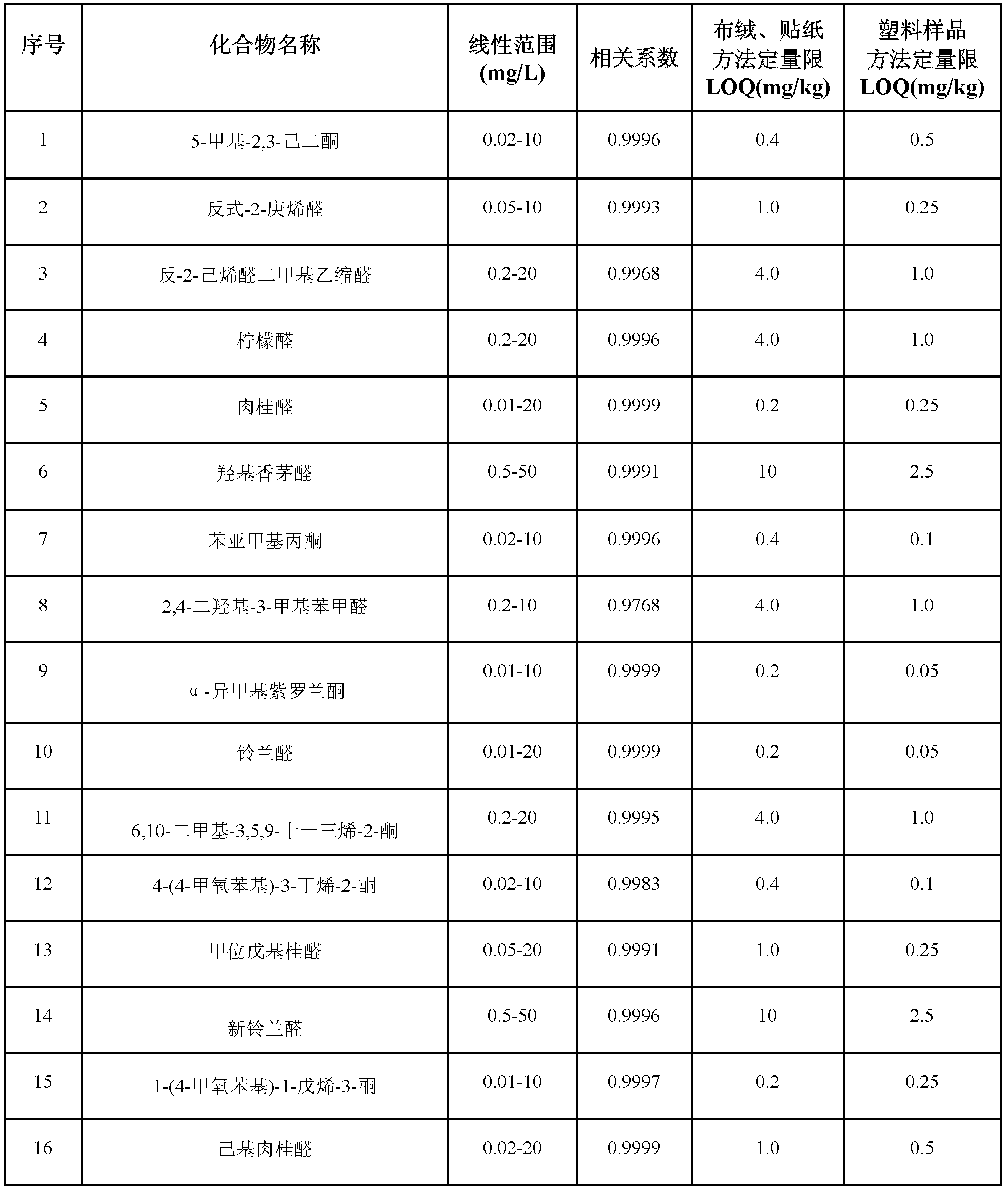
Method for simultaneously determining residual quantities of sixteen sensitized aldehyde and ketone perfumes in toy
The invention relates to a method for simultaneously determining residual quantities of sixteen sensitized aldehyde and ketone perfumes in a toy, and firstly discloses a method for simultaneously determining the residual quantities of the sixteen sensitized aldehyde and ketone perfumes consisting of 5-methyl-2,3-hexanedione, trans-2-heptenal, trans-2-hexenaldimethylacetal, citral, cinnaldehyde, hydroxycitronellal, benzalacetone, 2,4-dihydroxy-3-methylbenzaldehyde, alpha-isomethylionone, lilial, 6,10-dimethyl-3,5,9-undecatrien-2-one, 4-(4-methoxyphenyl)-3-butylene-2-one, alpha-amyl cinnamic aldehyde, lyral, 1-(4-methoxyphenyl)-1-pentene-3-one, and hexylcinnamaldehyde in the toy to fill the technological blank. The method which allows cloth toys, paster toys, and plastic toys (the ABS material, the PVC material and the PS material) to be detected has the advantages of wide coverage and strong applicability. The method which adopts the ion trap-mass spectrum in a qualitative and quantitative secondary mass spectrum MS / MS mode has a good qualitative and quantitative capability to complex matrix toy samples.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Cosmetic or dermatological preparation for prophylaxis and/or treatment of atopic dermatitis
ActiveUS9789099B2Cut skinReduce colonizationHydroxy compound active ingredientsAerosol deliveryAtopic dermatitisAgonist
Agonists for the TRPM-8 receptor, more particularly one or more substances selected from the group of, for example, linalool, geraniol, hydroxycitronellal, WS-3 (N-ethyl-p-menthane-3-carboxamide), WS-23 (2-isopropyl-N,2,3-trimethylbutyramide), Frescolat MAG (1,4-dioxaspiro[4.5]decane-2-methanol), Frescolat ML (menthyl lactate), Coolact P (5-methyl-2-prop-1-en-2-ylcyclohexan-1-ol) and Cooling Agent 10 (menthoxypropanediol), for use as medicaments to counter atopic dermatitis.
Owner:BEIERSDORF AG
Soybean paste and preparation method thereof
The invention provides soybean paste. The soybean paste is prepared from, by weight, 200-300 parts of soybeans, 180-200 parts of flour, 10-20 parts of dark plums, 12-20 parts of kiwi fruits and 5-12 parts of additive. The additive is prepared from, by weight, 1-4 parts of hydroxycitronellal dimethyl acetal, 2-4 parts of laurinol and 2-4 parts of raspberry ketone. The amino acid nitrogen content is high after the soybean paste is placed.
Owner:滁州先奇工业科技有限公司
High-efficiency mosquito-repelling skin care lotion and preparation method thereof
The invention discloses a high-efficiency mosquito-repelling skin care lotion and a preparation method thereof. The high-efficiency mosquito-repelling skin care lotion is characterized by consisting of the following raw materials: 3-5 percent of 10-20 percent of hydroxycitronellal 1,2-propanediol acetal, 2-5 percent of geranium extract, 5-10 percent of honey, 5-10 percent of egg white, 0.5-2 percent of Chinese angelica, 0.5-2 percent of peach kernel, 0.5-2 percent of salvia miltiorrhiza, 0.5-2 percent of radix angelicae, 0.5-2 percent of rhizoma typhonii, 0.5-2 percent of rhizoma bletillae and the balance of water. The preparation method comprises the following steps: adding water into the traditional Chinese medicine components for decocting, concentrating and cooling; and adding water into the egg white for diluting, mixing with the traditional Chinese medicine component concentrated solution, adding the 3-5 percent of 10-20 percent of hydroxycitronellal 1,2-propanediol acetal for emulsifying and forming. Compared with a conventional mosquito-repelling product, the mosquito-repelling emulsion has the high-efficiency and long-lasting beneficial effects, has the skin care and beauty effect and integrates the effects of repelling mosquitoes, relieving itching, protecting the skin and beautifying. Moreover, by adoption of the nontoxic raw materials which pass through GRAS (Generally Recognized as Safe) authentication, the product is safe and reliable.
Owner:刘桐言
Oriental flower flavored essence and preparation method thereof
ActiveCN112760167AGood quality improvementStrong aroma coordinationEssential-oils/perfumesAgainst vector-borne diseasesBenzoic acidFlavoring essences
The invention relates to the field of essence, and particularly discloses oriental flower flavored essence and a preparation method thereof. The oriental flowery flavor essence consists of a main flavor, a modifier, a mixing agent, a fixative and a solvent. The main flavor is prepared from the following components: linalool, benzyl alcohol, phenethyl alcohol, benzyl acetate, methyl anthranilate, methyl benzoate, methyl salicylate, iso-methyl ionone, alpha-hexylcinnamic aldehyde, anisic aldehyde, hydroxycitronellal, undecan-4-olide, specially pure vanillin, ethyl maltol, eugenol and limonene. The modifier is prepared from the following components: cinnamic alcohol, rose ether and cedar wood oil. The oriental flower flavored essence has the advantages that the oriental flower flavored essence has a comfortable and natural feeling while generating complicated fragrance change.
Owner:广州芬豪香精有限公司
Novel Shatian pomelo tea vinegar drink
The invention discloses a novel Shatian pomelo tea vinegar drink, which comprises the following raw materials: Shatian pomelo pulp, Shatian pomelo peel, golden camellia, nutmeg, dark plum, semen plantaginis, licorice, hovenia dulcis thumb, persimmon leaf, Chinese olive, semen lablab album, radix sophorae flavescentis, galangal, orange peel, amomum tsao-ko, grape vinegar, ginkgetin, potassium iodide, carboxymethylcellulose sodium, agar, polyvinyl sodium, sodium alginate, a DuPont EEA2112AC compatilizer, a food-grade antioxidant BHT, locust bean gum, carthamin yellow, nisin, trisodium glycyrrhizinate, glucose, fructose, Thaumatin, vitamin A, vitamin C, vitamin D, isoleucine, lysine, threonine, tryptophan, iron element, manganese element, chromium element, copper element, ethyl-furfuryl fumarate, potassium inosinate, potassium guanylate, sodium L-glutamate, 9-cycloheptadecanone, hydroxycitronellal, ionone and vanillin. The drink provided by the invention can effectively break down alcohol, thus relieving damage of alcohol to the nervous system, etc.
Owner:孔令娇
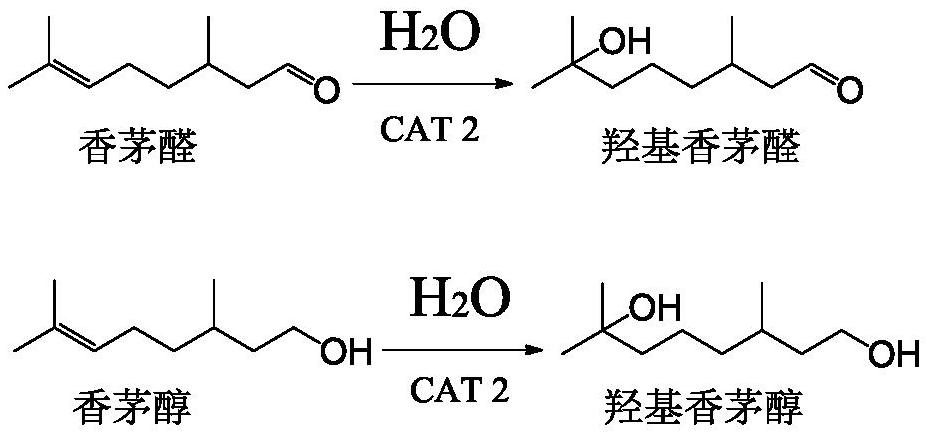
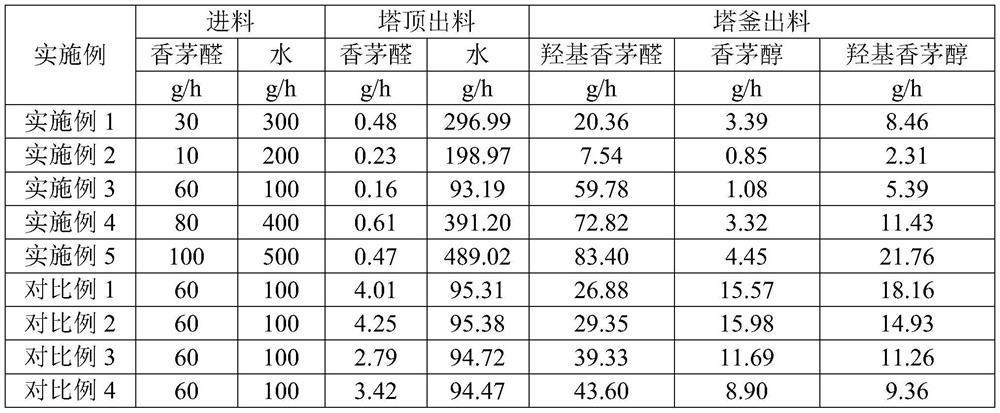
Method for co-producing hydroxycitronellal and hydroxycitronellol
ActiveCN112125792ARealize joint productionImprove responsePhysical/chemical process catalystsOrganic compound preparationHydration reactionHYDROXYCITRONELLOL
The invention discloses a method for co-producing hydroxycitronellal and hydroxycitronellol. The method comprises the following steps of: arranging a reactive rectification zone I, a common rectification zone and a reactive rectification zone II which are connected from top to bottom as a reactive rectification system, carrying out hydration reaction on citronellal and water in the reactive rectification zone II to prepare hydroxycitronellal, and carrying out hydrogenation reaction on citronellal and hydrogen in the reactive rectification zone I to generate citronellol, and bringing the citronellol into the reactive rectification zone II to be subjected to a hydration reaction to obtain hydroxycitronellol. Citronellal is fed from a tower kettle of the reactive rectification zone II; wateris fed from a tower kettle of the reactive rectification zone II; and hydrogen is fed from the common rectification zone. According to the method, citronellal is used as the raw material, and hydrogenation reaction, hydration reaction and rectification separation are combined to co-produce hydroxycitronellal and hydroxycitronellol. The technological process is greatly simplified, and the method has the advantages of high yield, few three wastes, high selectivity and the like.
Owner:WANHUA CHEM GRP CO LTD
Watermelon essence for oil-based ink and preparation method of watermelon essence
InactiveCN105132174AIncrease added valueEasy to useInksEssential-oils/perfumesSolubilityPhenethyl acetate
The invention relates to a watermelon essence for oil-based ink. The watermelon essence is prepared from watermelon essence, maltodextrin and starch sodium octenylsuccinate, wherein the watermelon essence is prepared from leaf alcohol, hexenyl acetate, ligustral, phenyl acetaldehyde dimethyl acetal, methyl heptenone, trans-2-hexenal, nonadienaldehyde, melonal, ethyl caprylate, ethyl isovalerate, hexyl acetate, allyl cyclohexanepropionate, benzyl alcohol, linalool, citronellol, geraniol, Alpha terpneol, phenethyl alcohol, hydroxycitronellal, lilial, heliotropin, citral, dimethylbenzylcarbinyl acetate, vanillin, alpha-damascenone, isomethyl ionone, verdyl acetate, linalyl acetate, linalyl acetate, styralyl acetate, phenethyl acetate, phenethyl isobutyrate, phenoxyethyl isobutyrate, terpinyl acetate, n-pentyl salicylate, benzyl benzoate, benzyl salicylate and Brazil orange oil. The invention also provides a preparation method for the watermelon essence for the oil-based ink. According to the watermelon essence and the preparation method, the fragrance retention effect and the oil solubility of the essence are improved.
Owner:SHANGHAI INST OF TECH
Melamine derivative-modified waterborne polyester flame retardant resin and preparation method thereof
InactiveCN110591062AStrong adhesionImprove fullnessFireproof paintsPolyester coatingsPolyesterPliability
The invention relates to melamine derivative-modified waterborne polyester flame retardant resin and a preparation method thereof. The melamine derivative-modified waterborne polyester flame retardantresin comprises the components: in parts by weight, 8.0-20.0 parts of organic acid anhydride, 5.0-10.0 parts of a hydroxycitronellal-modified melamine derivative, 2.0-5.0 parts of glycerol monolaurate, 2.0-5.0 parts of oligomer diol, 4.0-15.0 parts of polyols, 1.5-3.5 parts of 2,2-bipyridyl-5-carboxylic acid, 2.0-4.0 parts of dimethylolpropionic acid, 5.0-10.0 parts of national-standard xylene, 3.0-6.0 parts of a neutralizing agent, 0.2-0.5 part of an antioxidant and 45.0-60.0 parts of deionized water, wherein the hydroxycitronellal-modified melamine derivative is obtained through a reactionbetween melamine and hydroxycitronellal. The melamine derivative-modified waterborne polyester flame retardant resin has good adhesion, high hardness, good fullness, good flexibility, no environmentalpollution, good flame retardancy, low cost, simple construction and other features, and can be applied to coatings of inner walls and outer walls, wood coatings and other fireproof flame retardant coatings.
Owner:UNION FOSHAN CHEM +1
A Shatian pomelo beverage
InactiveCN106234871AFull of nutritionStrong tea fragranceFood ingredient as taste affecting agentNatural extract food ingredientsCelluloseVitamin C
A Shatian pomelo beverage is disclosed. Raw materials of the beverage comprises Shatian pomelo flesh, Shatian pomelo peel, camellia chrysantha, flower of lobed kudzu vine, chrysanthemum flower, hawthorn fruit, licorice, raisin tree seed, mint, mulberry fruit, white hyacinth bean, lightyellow sophora root, lesser galangal rhizome, tangerine peel, fruit of caoguo, grape vinegar, ginkgetin, potassium iodide, sodium carboxyethyl cellulose, agar, sodium polyacrylate, sodium alginate, an EEA2112AC compatilizer of American Dupont, a food-grade antioxidant BHT, locust bean gum, carthamin yellow, nisin, trisodium glycyrrhizinate, glucose, fructose, thaumatin, vitamin A, vitamin C, vitamin D, isoleucine, lysine, threonine, tryptophan, an iron element, a manganese element, a chromium element, a copper element, ethyl furfuryl fumarate, potassium inosinate, potassium guanylate, L-sodium glutamate, 9-cycloheptadecen-1-one, hydroxycitronellal, ionone and vanillin. The beverage can effectively decompose alcohol and relieve alcohol-caused harms on the nervous system and the like.
Owner:孔令娇
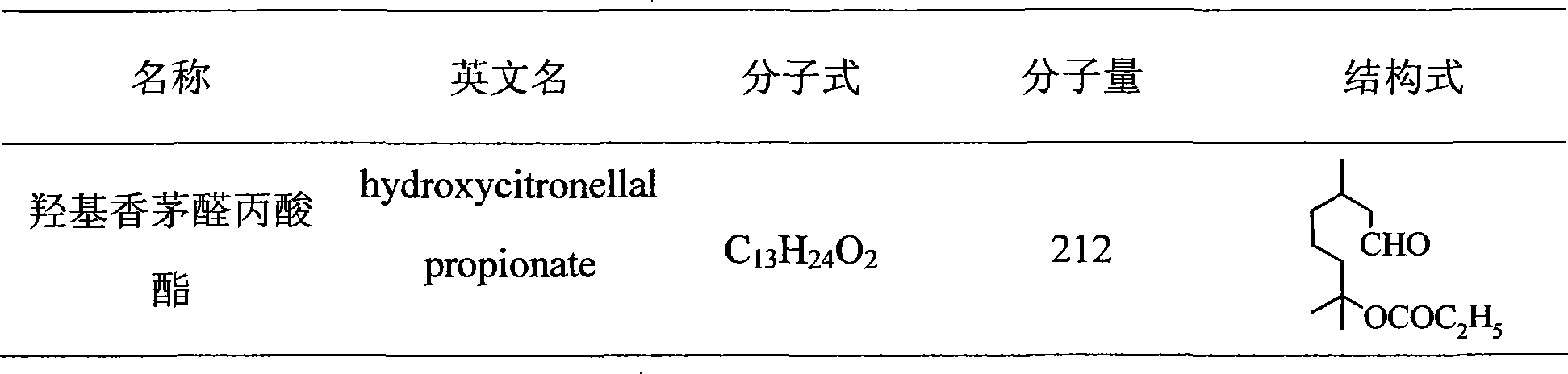
Application of hydroxycitronellal propionic ester used as insect repellant and antifeedant
InactiveCN101238810ALow densityTo achieve the purpose of protectionBiocidePest repellentsPropionateDiamondback moth
The invention relates to a kind of hydroxycitronellal propionate applied in insect repellent agent and antifeedant, including hydroxycitronellal propionate used as repellent agent of cockroach and red flour beetle, and hydroxycitronellal propionate used as antifeedant of diamondback moth larva. The bioassay result shows that, the repellent activity of 20 mg / ml hydroxycitronellal propionate against male Blattella germanica is up to 62%, the repellent activity of 100 mg / ml hydroxycitronellal propionate against female Blattella germanica is up to 65%. In field experiments, the hydroxycitronellal propionate also shows good effective. The repellent activity of hydroxycitronellal propionate against red flour beetle is also high, at 8mg / ml, after 12, 24, 72 hours, the repellent rate is above 90%; the antifeedant activity of the compound against diamondback moth larva is high, at 10 mg / ml, after 24, 48, 72 hours, the antifeedant rate is 86%, 77%, 67% respectively.
Owner:江苏敖广日化集团股份有限公司
Jasmine-fragrance-type lipstick and preparation method thereof
InactiveCN102697700ABright colorHigh transparencyCosmetic preparationsMake-upBiotechnologyCinnamic aldehyde
The invention belongs to the technical field of cosmetics of daily necessities. A jasmine-fragrance-type lipstick is characterized by being prepared from four raw materials including a component A, a component B, a component C and a component D in proportion by weight of 3 to 82 to 14 to 1, wherein the component A is titanium dioxide; the component B consists of castor oil, wool alcohol, wool ester, hydrogenated animal oil, vaseline, carnauba wax, paraffin, propylparaben, benzene methanol and tertiary butyl hydroxy benzoic ether; the component C consists of hydrolyzed animal protein, calendula immersion liquid and pigment; and the component D consists of alpha-amyl cinnamic aldehyde, benzyl acetate, benzyl salicylate, phenylethyl alcohol, nerol, benzyl alcohol, indole, jasmine net oil, orange leaf oil, neroli, alpha-lonone, hydroxyl citronellal and butyl butyrate. The invention also discloses a preparation method of the jasmine-fragrance-type lipstick. The jasmine-fragrance-type lipstick and the preparation method of the jasmine-fragrance-type lipstick have the beneficial effects that the lipstick product is brilliant in color, is bright, and is high in transparency, and has light jasmine fragrance. The preparation method is simple, and raw materials are easy to purchase.
Owner:马建欣
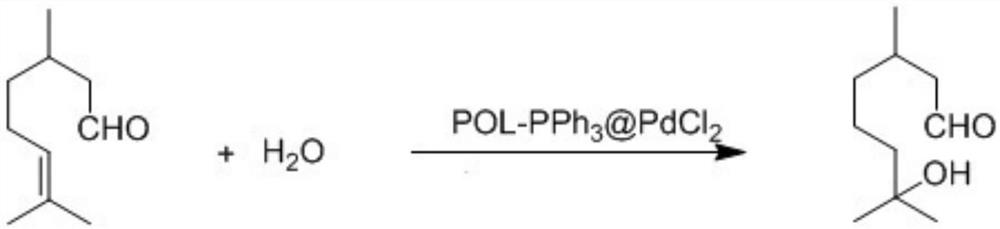
Efficient green synthesis method of hydroxycitronellal
ActiveCN113024364AHigh catalytic efficiencyHigh yieldOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystEngineering
The invention discloses an efficient green synthesis method of hydroxycitronellal. The method comprises the following steps: adding POL-PPh3, PdCl2 and anhydrous THF into a sealed container according to a mass ratio of (100: 5)-(10: 1), reacting at 25-35 DEG C overnight, filtering, removing filtrate, washing filter residues twice with ethyl acetate and ethanol respectively, and collecting solids to obtain a POL-PPh3-coated PdCl2 solid catalyst; taking 1 part by mass of a POL-PPh3-coated PdCl2 catalyst, 10-35 parts by mass of citronellal, 7-20 parts by mass of water and 500-800 parts by mass of toluene, uniformly stirring, and reacting at 180-200 DEG C for 10-24 hours to obtain a reaction solution; after the reaction liquid is cooled, filtering and separating the solid catalyst, fractionating the reaction liquid, and collecting a distillate at 257 DEG C, thereby obtaining the hydroxycitronellal. According to the method, citronellal and water are directly synthesized into hydroxycitronellal in one step under the catalysis of POL-PPh3-coated PdCl2, the method has the advantages of being environmentally friendly, low in price, high in catalytic efficiency, high in yield, mild in reaction condition, high in production efficiency, simple in process, non-toxic, harmless, free of emission of three wastes and the like, and a good foundation is laid for large-scale production of hydroxycitronellal.
Owner:GUANGXI FORESTRY RES INST +1
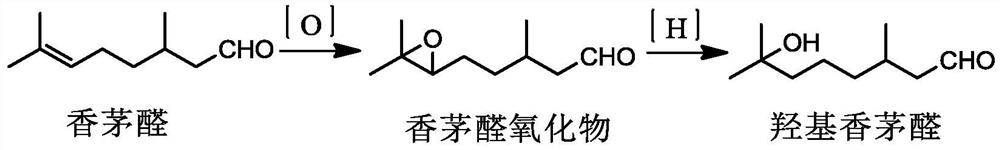
Method for preparing citronellal epoxide
ActiveCN113005472AMild reaction conditionsHigh selectivityElectrolysis componentsElectrolytic organic productionElectrolytic agentPtru catalyst
The invention discloses a method for preparing citronellal epoxide. The method comprises the following steps: preparing an electrolyte containing citronellal, water, lower alcohol and cyclodextrin or a derivative thereof, standing, transferring into an electrolytic bath with a cathode and an anode, and carrying out electrochemical oxidation. The raw material citronellal is subjected to high-selectivity oxidation by using an electrochemical oxidation method to obtain the citronellal epoxide, and the epoxide can be further subjected to hydrogenation ring opening to obtain important essence and perfume hydroxycitronellal. According to the method, no catalyst is used, the reaction condition is mild, the selectivity is high, and few reaction byproducts are generated.
Owner:WANHUA CHEM GRP CO LTD
Exterior wall paint
The present invention discloses an exterior wall paint, which is made from the following raw materials by weight: 2.5-7.6 parts of sticky powder, 3.5-8.2 parts of borax, 2.5-6.1 parts of polyisocyanate, 10-15 parts of titanium dioxide, 20-30 parts of white latex, 1.2-3.6 parts of hydroxy citronellal, 2.4-6.5 parts of dibenzyl diphenol polyoxyethylene ether, and 1.6-4.3 parts of acrylic resin polyurethane copolymer resin. Compared with the products of the prior art, the paint provided by the invention has strong cohesiveness, low price of raw materials, and greatly reduced production cost; besides, the paint has the characteristics of convenient usage, and the usage method is the same as that of a general commercial white latex paint. The exterior wall paint also has the substantial characteristics of simple process, long protection time on the object to be coated, and convenience for construction and application. The product can be widely used in building products, paint, paper, printing, furniture manufacturing, and other industries.
Owner:QINGDAO JINLIANXIN BUSINESS & TRADE
Novel outer wall paint
The invention discloses novel outer wall paint, which is prepared from the following raw materials in parts by weight: 2.5-7.6 parts of lime plaster, 3.5-8.2 parts of sodium borate, 2.5-6.1 parts of ethyl 2,2-dimethylpropionate, 10-15 parts of titanium dioxide, 20-30 parts of white emulsion, 1.2-3.6 parts of hydroxycitronellal, 2.4-6.5 parts of pesticide emulsifier 300#, and 1.6-4.3 parts of beta-1-N,N-dimethylin-2-propanolamine. Compared with an existing product, the novel outer wall paint has the advantages that stronger cohesive property is realized, the raw material price is low, and the production cost is also greatly reduced; the paint also has the characteristic of convenience in use, and an application method is the same as the application method of emulsion varnish sold on the common market. The novel outer wall paint also has the remarkable characteristics of simple process, long protection time for coated objects, convenience in construction, popularization and application, and the like. The product can be widely applied to multiple industries such as building, coating, papermaking, printing, and furniture manufacturing.
Owner:QINGDAO XIANGJIA INTPROP SERVICE CO LTD
Monomorium pharaonis repellent containing hydroxycitronellal diethylacetal
The invention discloses a monomorium pharaonis repellent containing hydroxycitronellal diethylacetal. The monomorium pharaonis repellent comprises the following raw materials in mass percent concentration: 0.2%-20% of hydroxycitronellal diethylacetal and 80%-99.8% of absolute ethyl alcohol; and when the mass percent concentration of hydroxycitronellal diethylacetal is 1%, the repelling rate on monomorium pharaonis is 100%. According to the monomorium pharaonis repellent, the hydroxycitronellal diethylacetal has a good repelling activity on the monomorium pharaonis and does not cause threat on the health of people and animals; the density of the monomorium pharaonis in a protected region can be effectively controlled and the harms are reduced; utilization regions and dosages of pesticides are reduced; and the monomorium pharaonis repellent has important meanings on prevention and treatment of the harms on the monomorium pharaonis.
Owner:JIANGXI AGRICULTURAL UNIVERSITY
Antiperspirant dew
InactiveCN102885712AQuickly decomposes toxic substancesReduce excessive sweat odorCosmetic preparationsToilet preparationsVitamin CChemical products
The invention relates to anantiperspirant dew, belonging to the technical field of daily chemical products. The anantiperspirant dew comprises components of propylene glycol, polysorbate, benzyl alcohol, hydroxycitronellal, magnesium chloride, vitamin C, essence, methylionone, aluminum chloride, magnesium nitrate and deionized water. The anantiperspirant dew is prepared by blending the above components according to a certain proportion. The anantiperspirant dew is not added with alcohol and other harmful substances during a preparation process, and is mild and non-stimulating. The anantiperspirant dew can effectively weaken much too heavy sweat smell caused by sweaty wet, rapidly decompose toxic substance in the sweat, and balance pH of part skin after being used, people feeling dry, comfortable and cool, and is suitable for easily-sweating and over-sweating people to use, being quite ideal in effect.
Owner:张宇
Jasmine-fragrance-type lipstick and preparation method thereof
InactiveCN102697700BBright colorHigh transparencyCosmetic preparationsMake-upBiotechnologyCinnamic aldehyde
The invention belongs to the technical field of cosmetics of daily necessities. A jasmine-fragrance-type lipstick is characterized by being prepared from four raw materials including a component A, a component B, a component C and a component D in proportion by weight of 3 to 82 to 14 to 1, wherein the component A is titanium dioxide; the component B consists of castor oil, wool alcohol, wool ester, hydrogenated animal oil, vaseline, carnauba wax, paraffin, propylparaben, benzene methanol and tertiary butyl hydroxy benzoic ether; the component C consists of hydrolyzed animal protein, calendula immersion liquid and pigment; and the component D consists of alpha-amyl cinnamic aldehyde, benzyl acetate, benzyl salicylate, phenylethyl alcohol, nerol, benzyl alcohol, indole, jasmine net oil, orange leaf oil, neroli, alpha-lonone, hydroxyl citronellal and butyl butyrate. The invention also discloses a preparation method of the jasmine-fragrance-type lipstick. The jasmine-fragrance-type lipstick and the preparation method of the jasmine-fragrance-type lipstick have the beneficial effects that the lipstick product is brilliant in color, is bright, and is high in transparency, and has light jasmine fragrance. The preparation method is simple, and raw materials are easy to purchase.
Owner:马建欣
Hydrophilic moisturizing mask
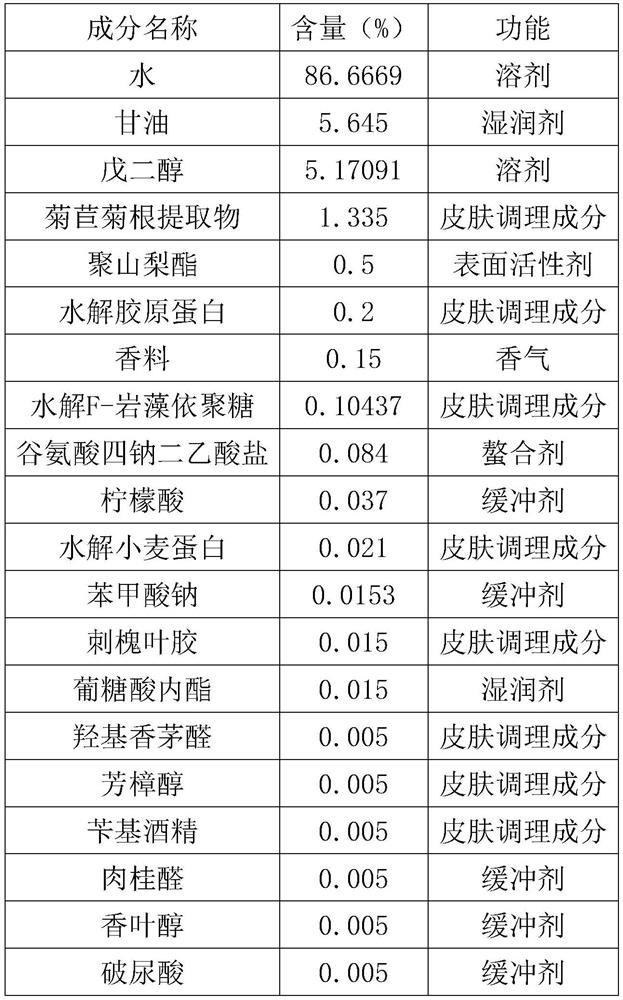
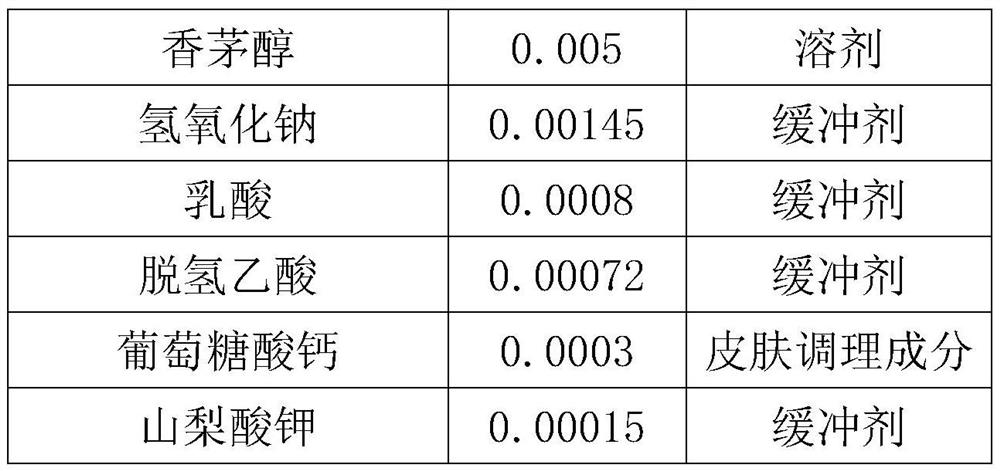
InactiveCN111700812AGood moisturizing effectNo damageCosmetic preparationsToilet preparationsDehydroacetic acidGlycerol
The invention provides a hydrophilic moisturizing mask characterized by comprising the following substances: water, glycerin, pentanediol, a chicory root extract, polysorbate, hydrolyzed collagen, xanthan gum, fragrance, hydrolyzed F-fucoidan, disodium glutamate tetraacetate, citric acid, hydrolyzed wheat protein, sodium benzoate, locust leaf gum, gluconolactone, hydroxycitronellal, linalool, benzyl alcohol, cinnamaldehyde, geraniol, hyaluonic acid, citronellol, sodium hydroxide, lactic acid, dehydroacetic acid, calcium gluconate, potassium sorbate. The hydrophilic moisturizing mask does not contain preservative components, does not cause irreversible damage to the skin of a user and has a good moisturizing effect.
Owner:玮格沃德贸易有限公司
Essence
InactiveCN103013659AReasonable formulaUnique ylang-ylang flavorEssential-oils/perfumesPropionateFormate
The invention discloses an essence. The essence is characterized by comprising the following components by weight percent: 17% of benzyl alcohol, 15% of benzyl acetate, 13% of benzyl benzoate, 14% of citronellol, 5% of ionone, 4% of linalyl acetate, 3% of benzyl propionate, 3% of benzyl propionate, 3% of amyl cinnamic aldehyde diphenyl acetal, 3% of phenethyl alcohol, 2% of amyl cinnamic aldehyde Schiff base, 2% of hydroxycitronellal, 2% of methyl eugenol, 10% of michelia alba leaf oil, 2% of fennel oil and 2% of benzyl formate. In an embodiment 1, the essence comprises the following components by weight percent: 17% of benzyl alcohol, 15% of benzyl acetate, 13% of benzyl benzoate, 14% of citronellol, 5% of ionone, 4% of linalyl acetate, 3% of benzyl propionate, 3% of benzyl propionate, 3% of amyl cinnamic aldehyde diphenyl acetal, 3% of phenethyl alcohol, 2% of amyl cinnamic aldehyde Schiff base, 2% of hydroxycitronellal, 2% of methyl eugenol, 10% of michelia alba leaf oil, 2% of fennel oil and 2% of benzyl formate.
Owner:张兆雷
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com