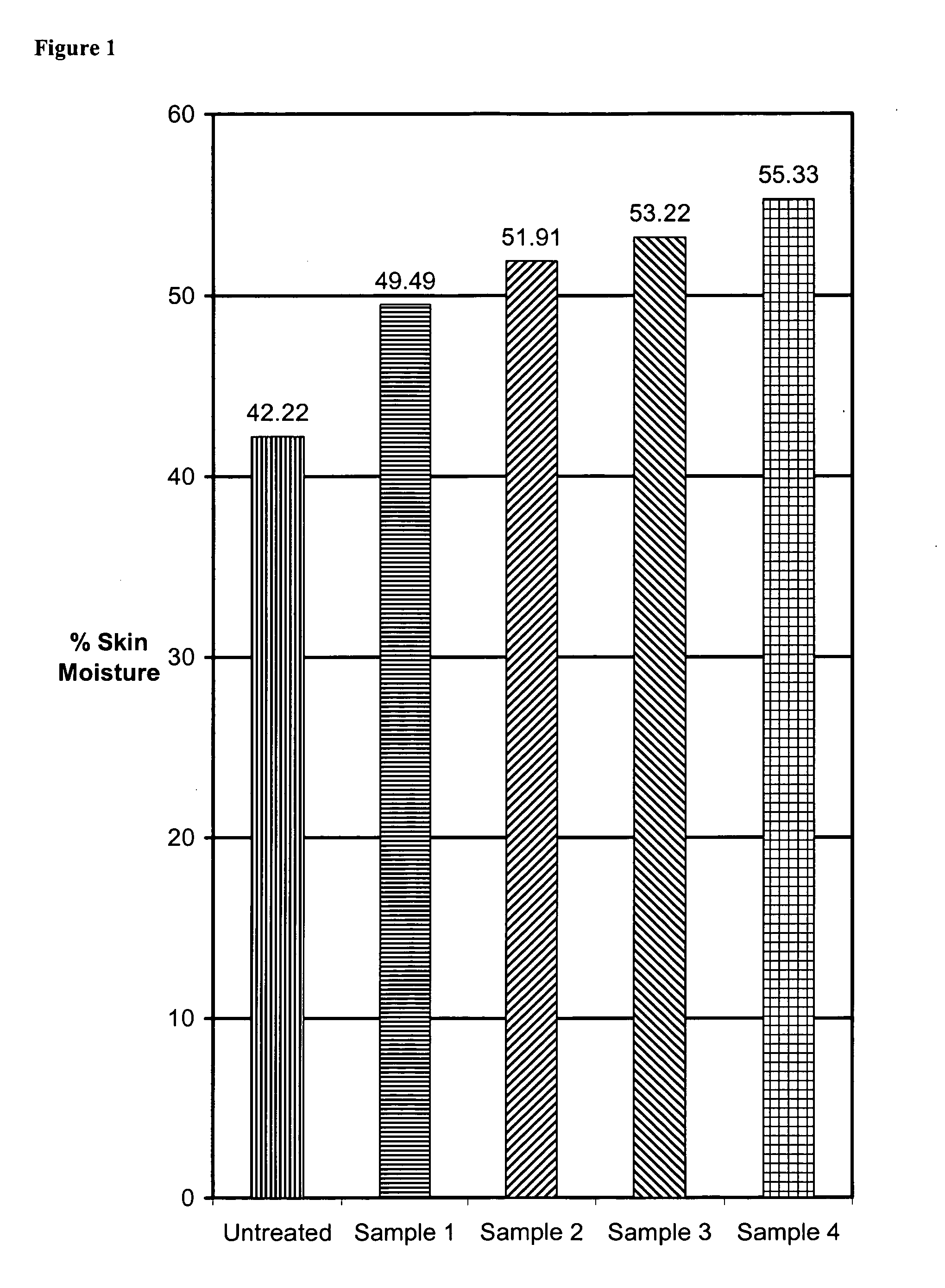
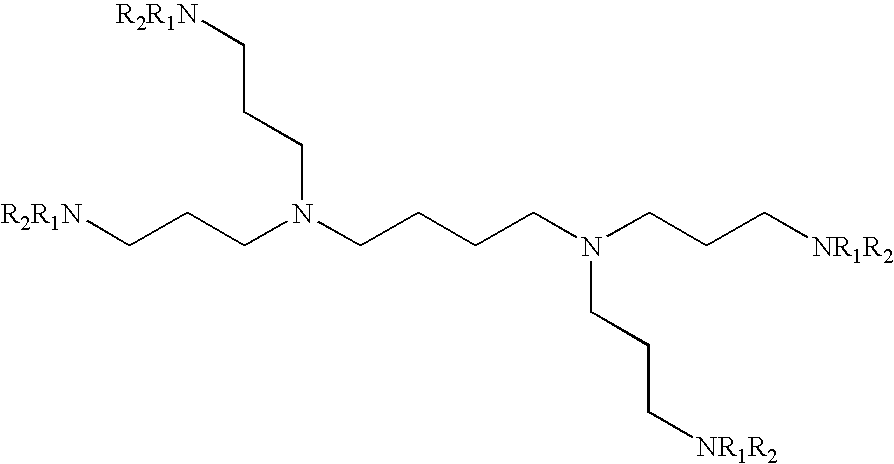
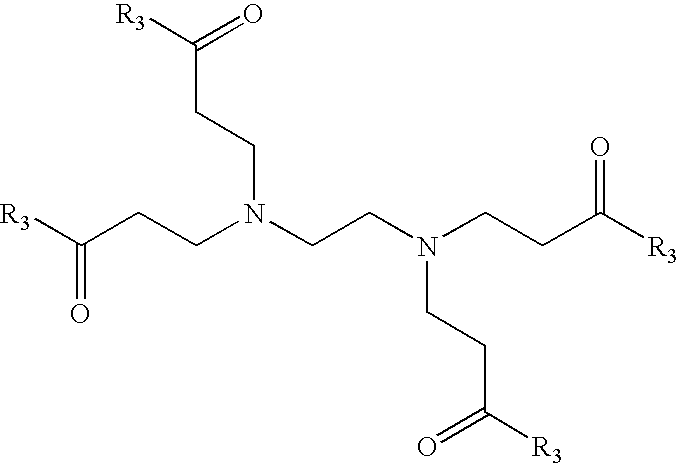
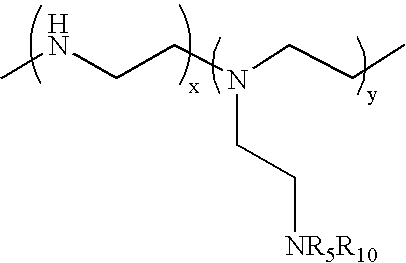
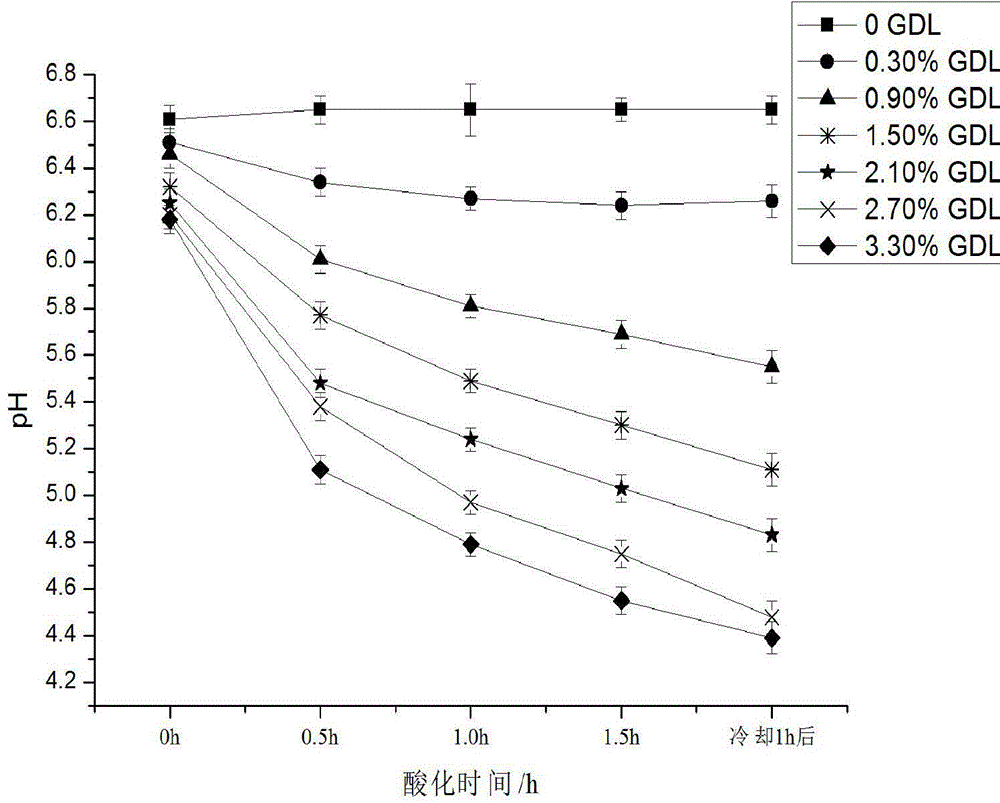
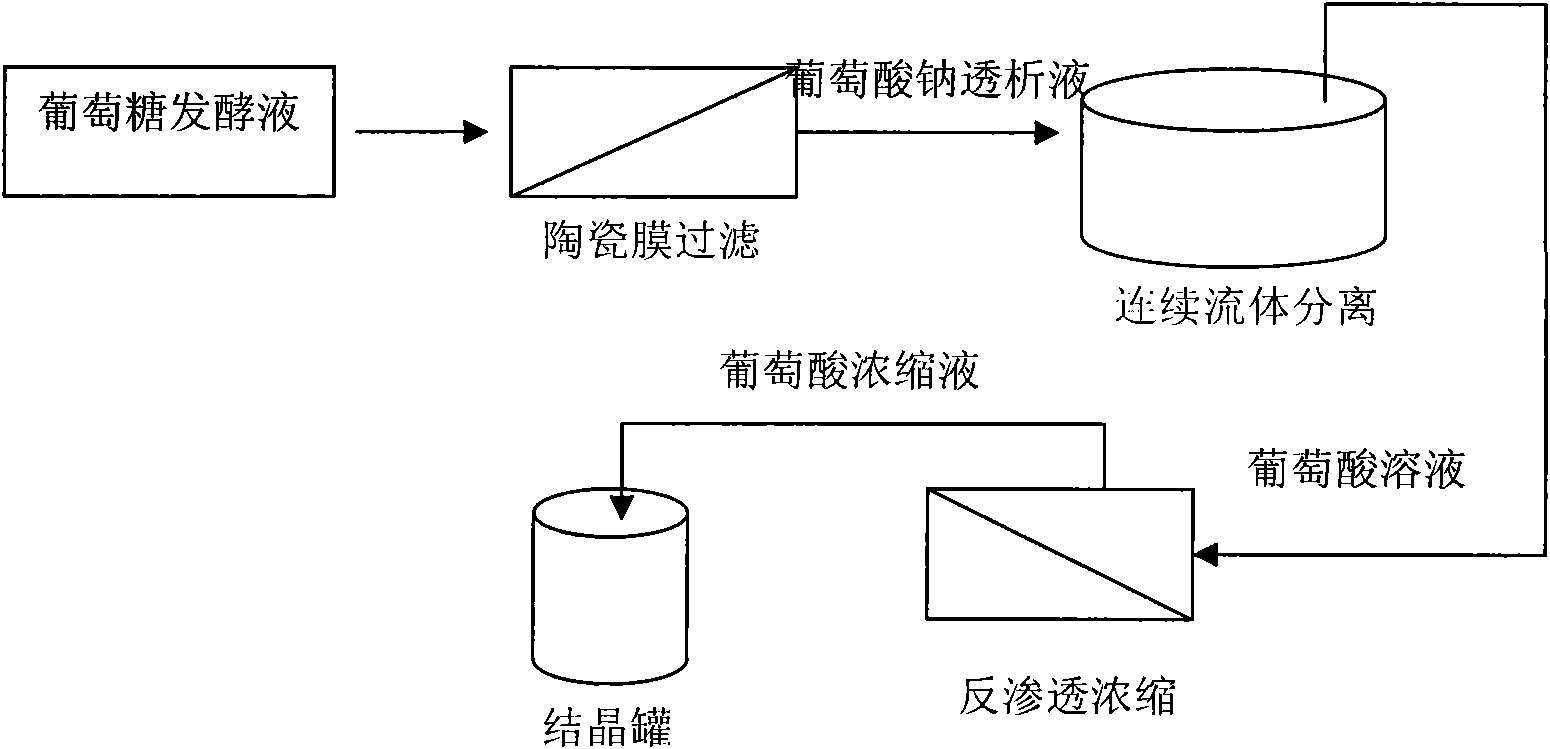
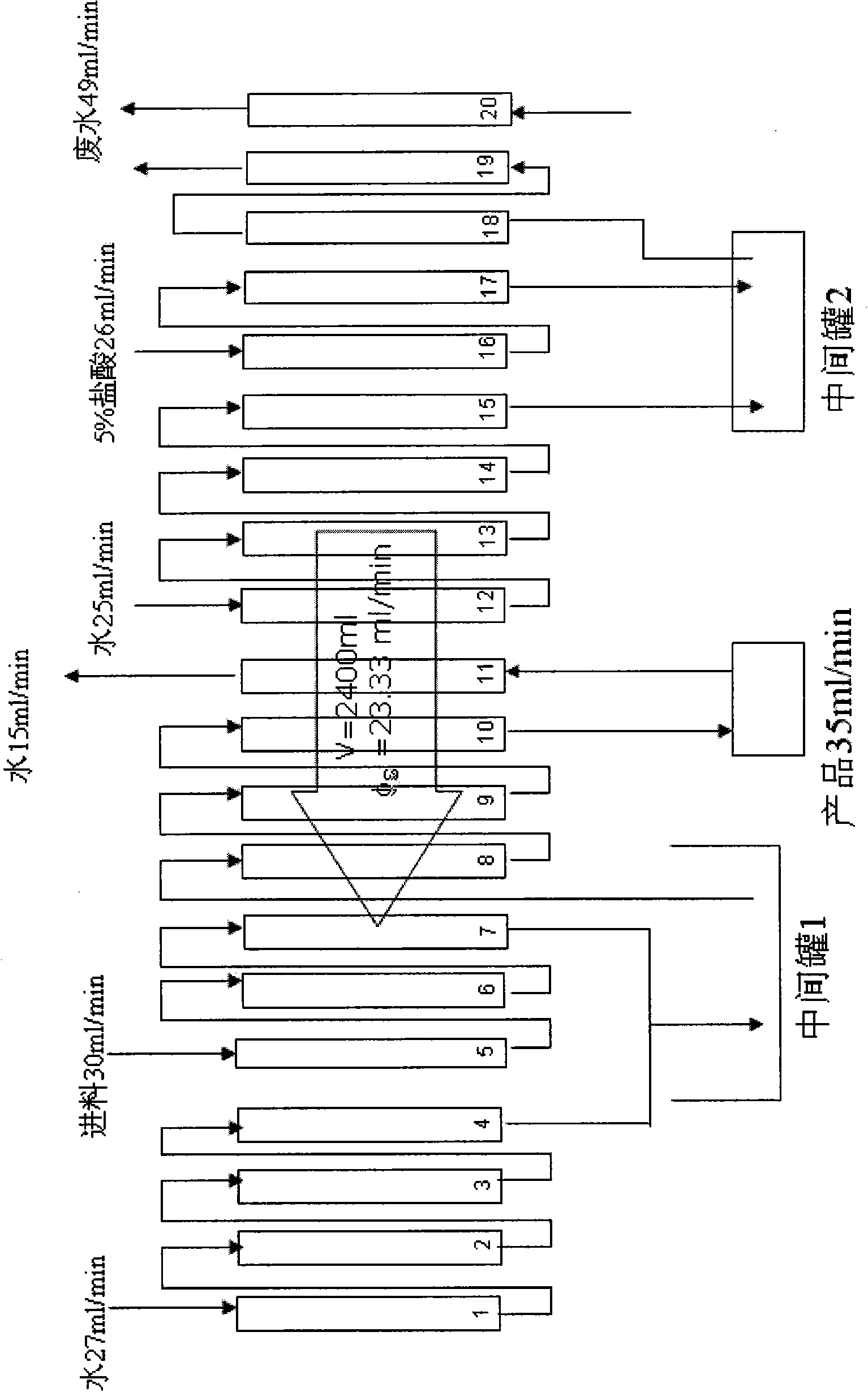
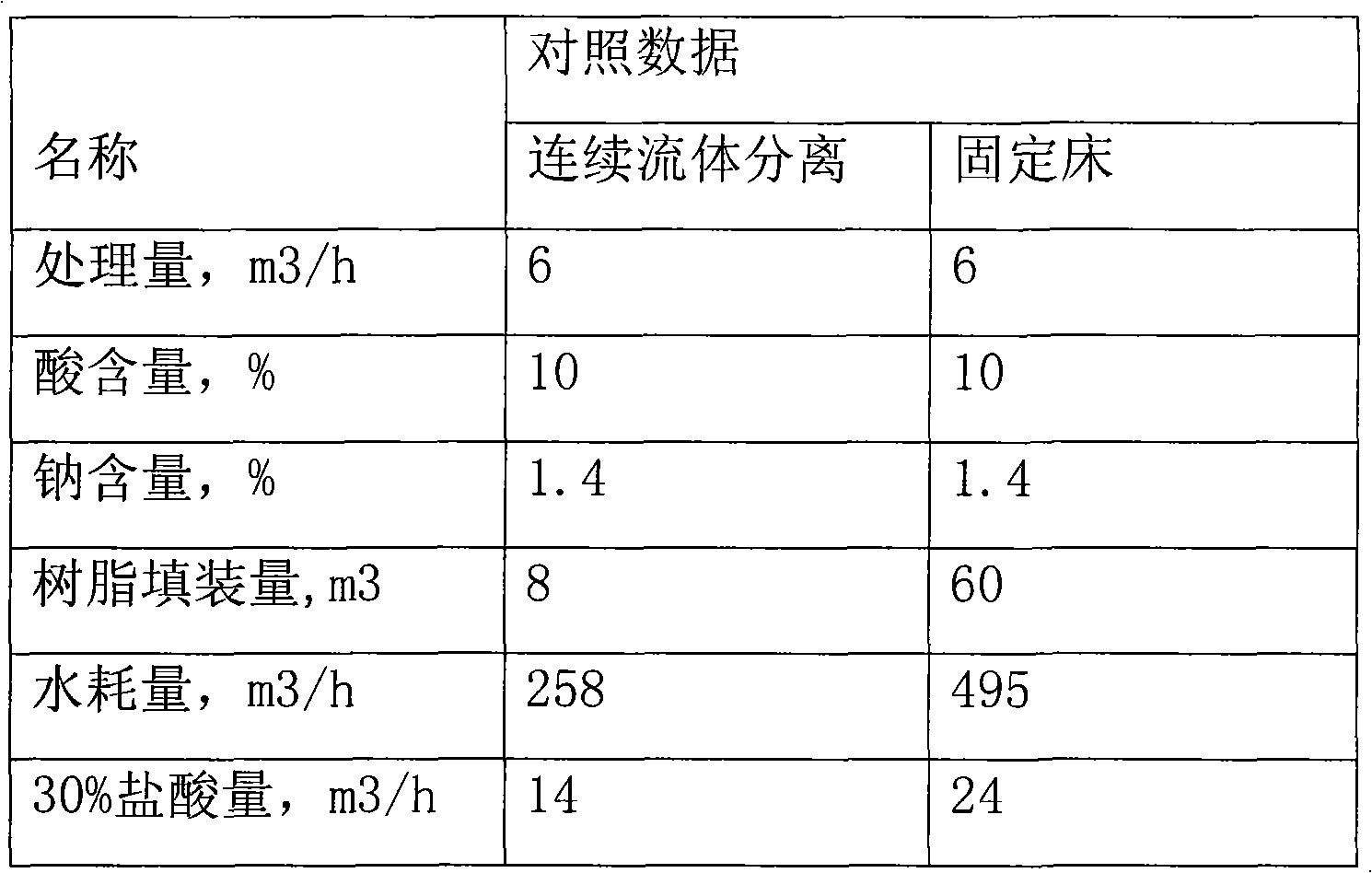
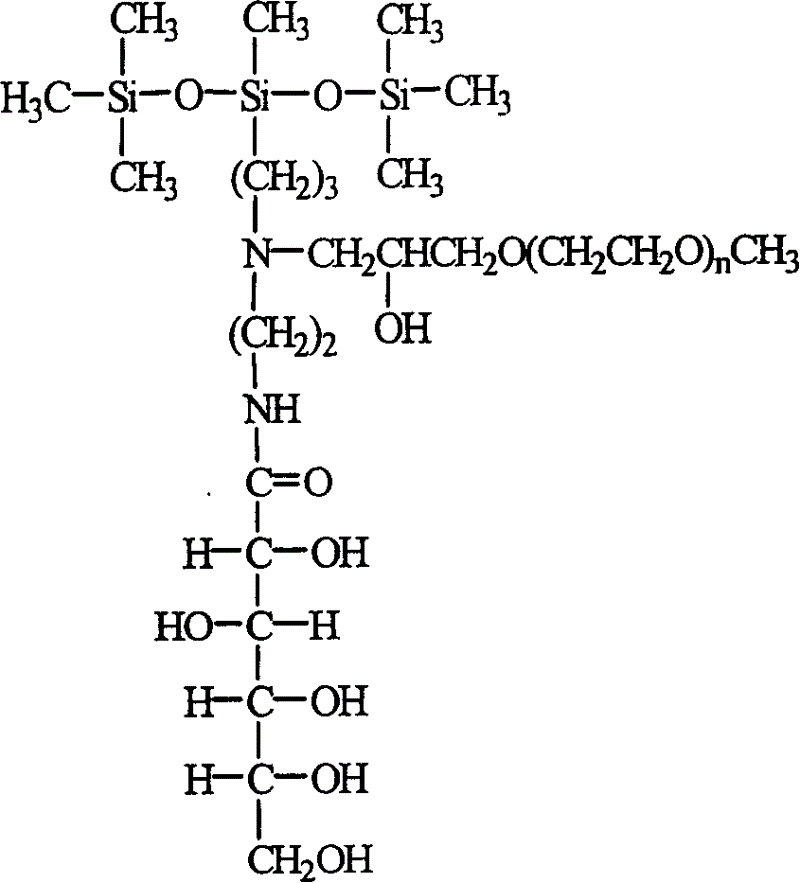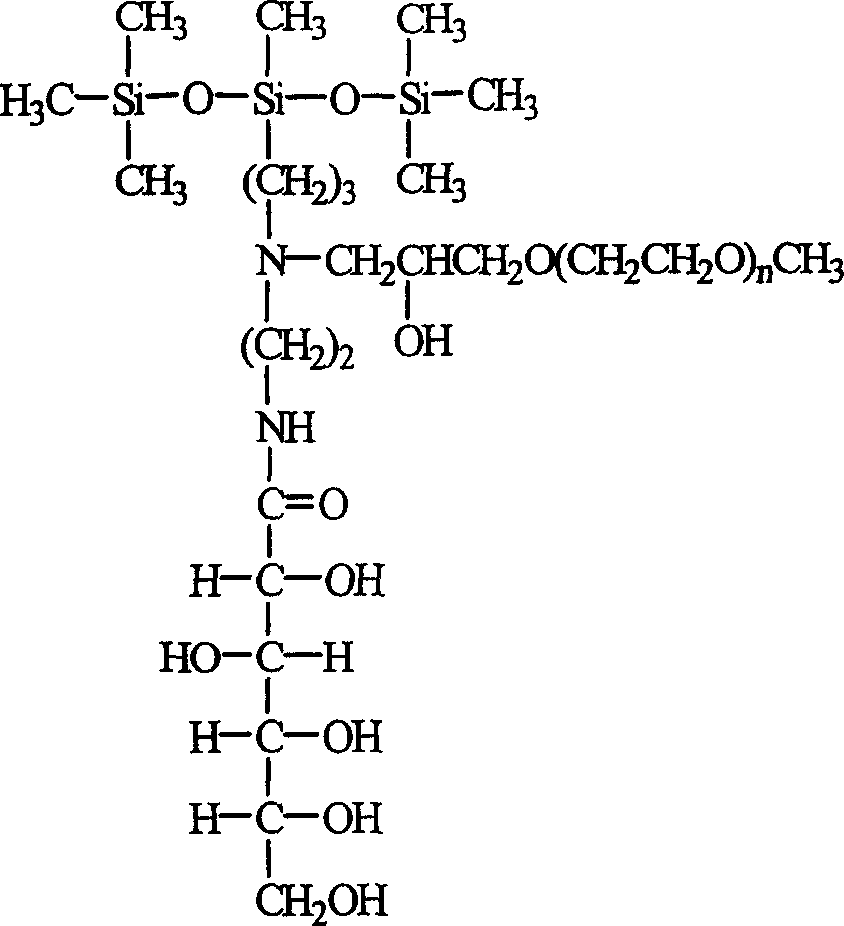Patents
Literature
463 results about "Gluconolactone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A naturally occurring polyhydroxy acid (PHA) with metal chelating, moisturizing and antioxidant activity. Gluconolactone can be produced by enzymatic oxidation of D-glucose oxidation. Its ability in free radicals scavenging accounts for its antioxidant property. It is used in cosmetic product and as a coagulant in tofu processing. (NCI05)
Personal care compositions and concentrates for making the same
InactiveUS20060093634A1Moisturize skin more effectivelyImprove biodegradation prevention efficacyCosmetic preparationsHair cosmeticsPersonal careMedicine
The present invention relates to color stable personal care compositions that provide anti-oxidiation, moisturization, and / or prevent biodegradation in personal care formulations. These compositions contain erythorbic acid or a salt thereof, caffeic acid or a salt thereof, δ-gluconolactone, or a mixture thereof. The present invention further relates to methods of moisturizing skin with erythorbic acid or a salt thereof, or δ-gluconolactone. The present invention also relates to concentrates of active ingredients preloaded with a sufficient amount of an anti-oxidation, moisturizing and / or biodegradation prevention agent so that when it is diluted for use, the final formulation includes an anti-oxidation, moisturizing and / or biodegradation prevention effective amount of the anti-oxidation, moisturizing, and / or biodegradation prevention agent.
Owner:LONZA INC
Method of clarifying oily waste water
ActiveUS20060289359A1Waste water treatment from quariesTreatment involving filtrationDemulsifierSulfate
A method of clarifying oily waste water comprising adding to the waste water an effective clarifying amount of one or more demulsifiers selected from the group consisting of dendritic polyamines, dendritic polyamidoamines and hyperbranched polyethyleneimines and the reaction products thereof with gluconolactone, alkylene oxides, salts of 3-chloro-2-hydroxypropanesulfonic acid, alkyl halides, benzyl halides and dialkyl sulfates.
Owner:ECOLAB USA INC
Regulation and control method of improving surimi system unfreezing forming and 3D (three dimensional) accurate printing performance
InactiveCN106798263AModerate viscosityImprove liquidityAdditive manufacturing apparatusFood ingredientsEngineeringGluconic acid
The invention discloses a regulation and control method of improving surimi system unfreezing forming and 3D (three dimensional) accurate printing performance and belongs to the technical field of preparation of 3D printed food materials. The regulation and control method comprises the following steps: unfreezing, mixing, blending, pre-forming, printing, post-setting, cooking frozen surimi and the like. According to the regulation and control method, as for an unfrozen surimi gel system, the printing temperature can be lowered, and the discharge rate and the stereo forming rate are increased; through induction by flaxseed gum and gluconic acid lactonic acid, the surimi gel has stable viscosity and mobility, surimi slurry is fine and smooth, and discharge is smooth without blockage; the surimi gel system can be prepared into fish product dining which maintains original characteristics and taste of a surimi product and has novel appearance and beauty through a 3D accurate printing technology.
Owner:JIANGNAN UNIV
Method for preparing oxane trisilicate of containing glucosyacylamino
InactiveCN1660882AAvoid toxicityAvoid the hydrosilylation reaction stepSugar derivativesDisiloxaneSolvent
A process for preparing trisiloxane containing glucosylamido used as the assistant of agricultural chemical to decrease the surface tension of water includes catalytic reaction between hexamethyl disiloxane and aminosilane to obtain aminotrisiloxane, and reacting on lacton gluconate in methanol.
Owner:CHINA RES INST OF DAILY CHEM IND
Minced fillet product producing method
ActiveCN103027317AReduce consumptionReduce manufacturing costFood preparationAquatic productFresh fish
A minced fillet product producing method relates to aquatic product processing and comprises the following steps: grinding without any addictive: adding a protease inhibitor and an acid seasoning aqueous solution into semi-thawed minced fillet, and chopping, blending, grinding and mixing uniformly at 4-10 DEG C; grinding with salt: adding salt and glucolactone into the ground minced fillet, grinding at 4-10 DEG C; gelating: shaping and heating the ground minced fillet, and gelating the minced fillet; and refrigerating: packaging in a sealing way, and refrigerating at 4-10 DEG C to obtain a minced fillet product. Only medium-temperature heating is required, so that the energy consumption is reduced and the production cost is reduced; the minced fillet product producing method is simple in process, low in requirement on equipment, short in production period and high in production efficiency; and the produced minced fillet product is faintly acid, low in fishy taste, strong in fresh fish meat taste, more compact in internal structure and macroscopically dedicate in mouth feel, and is superior in gel strength to he conventional minced fillet product heated at a high temperature.
Owner:SHISHI ZHENGYUAN AQUATIC TECH DEV
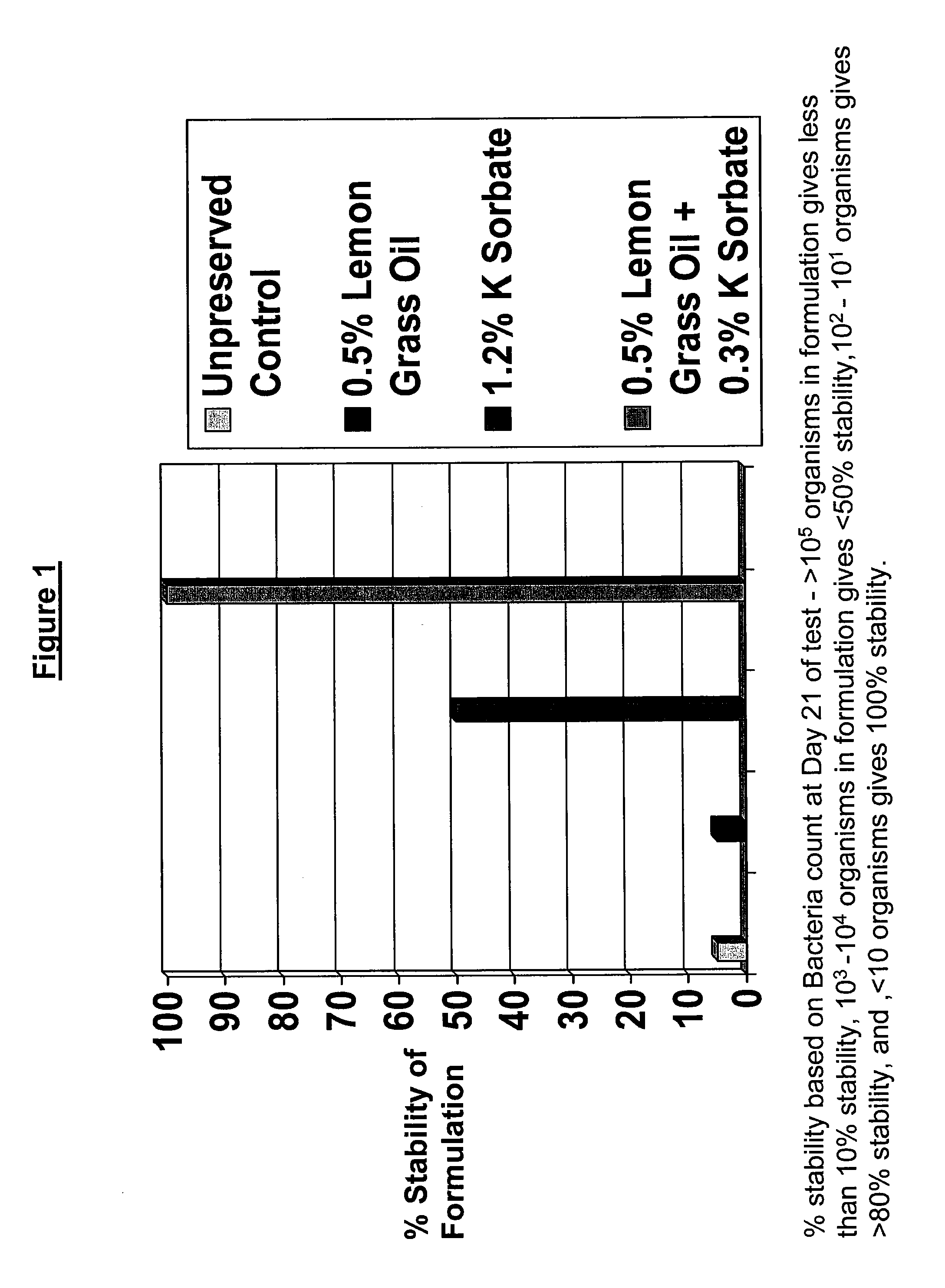
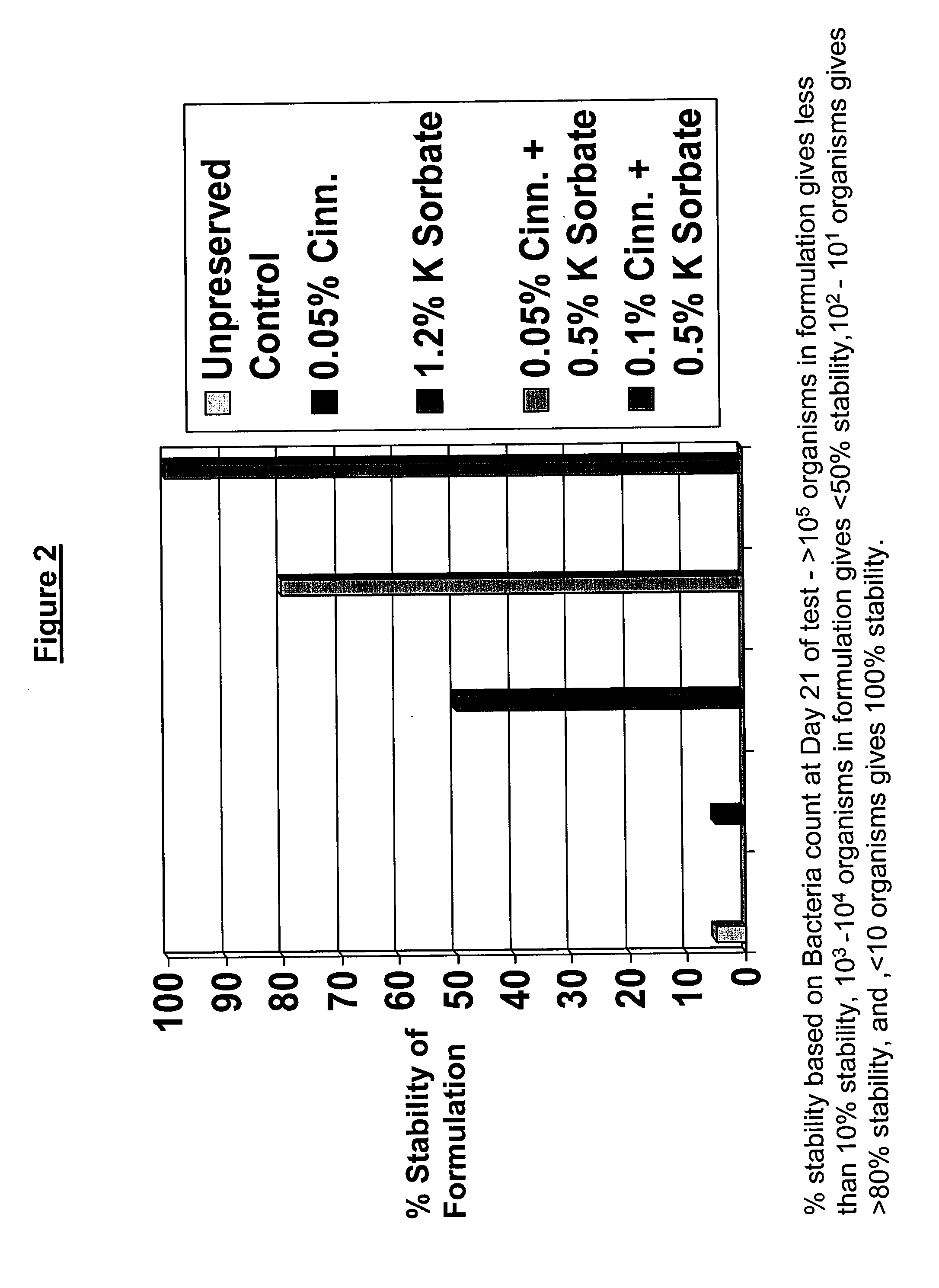
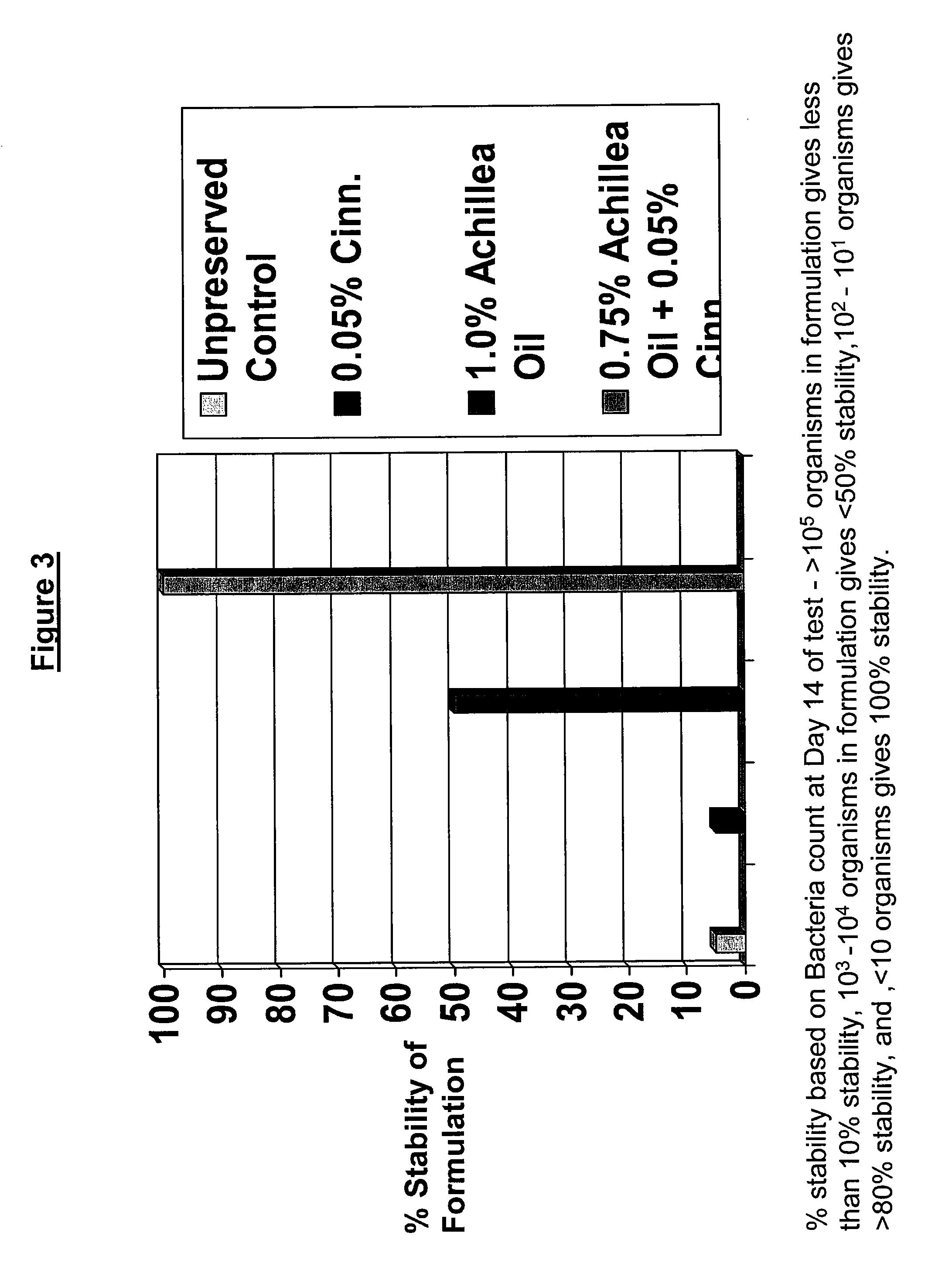
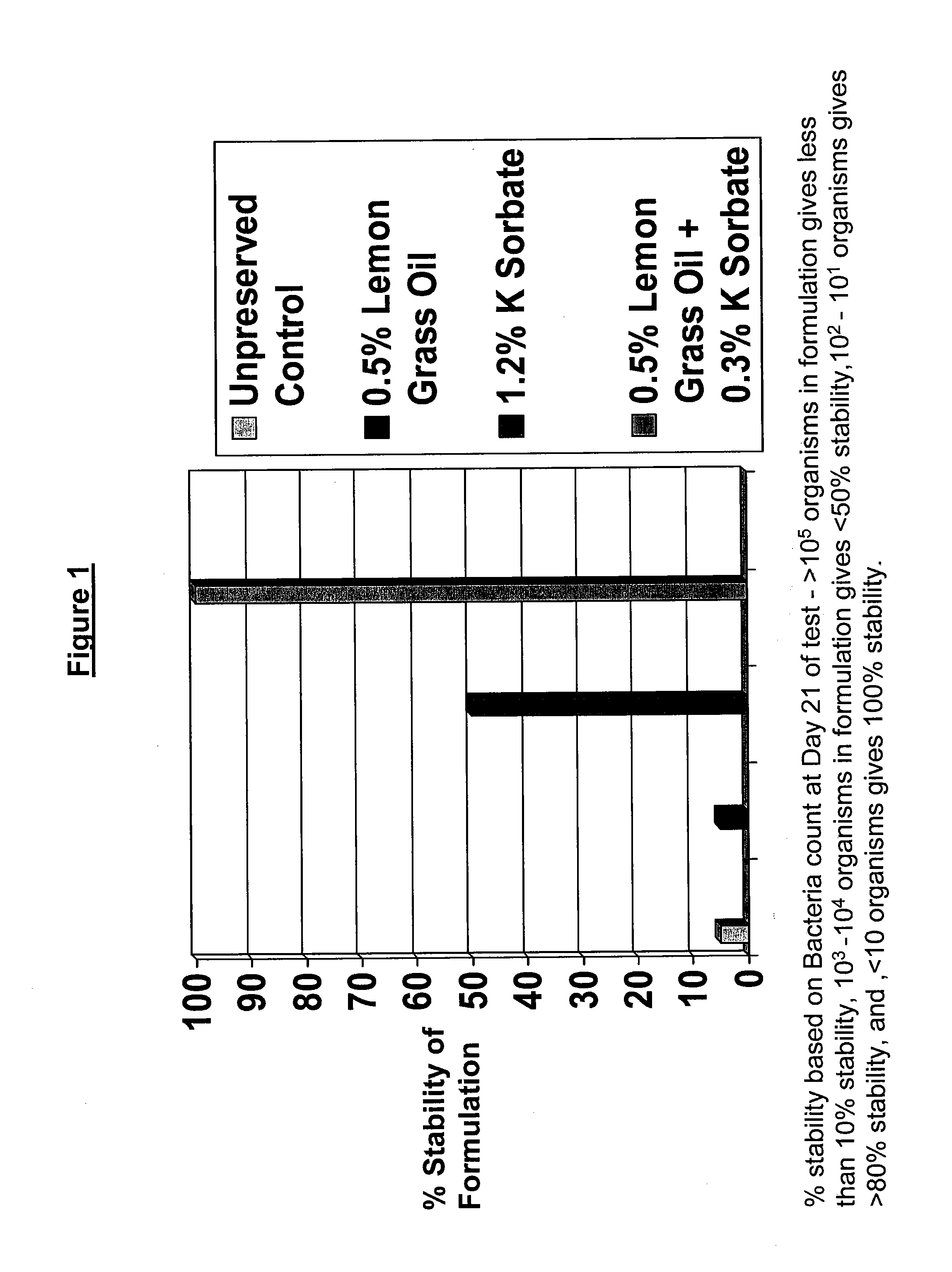
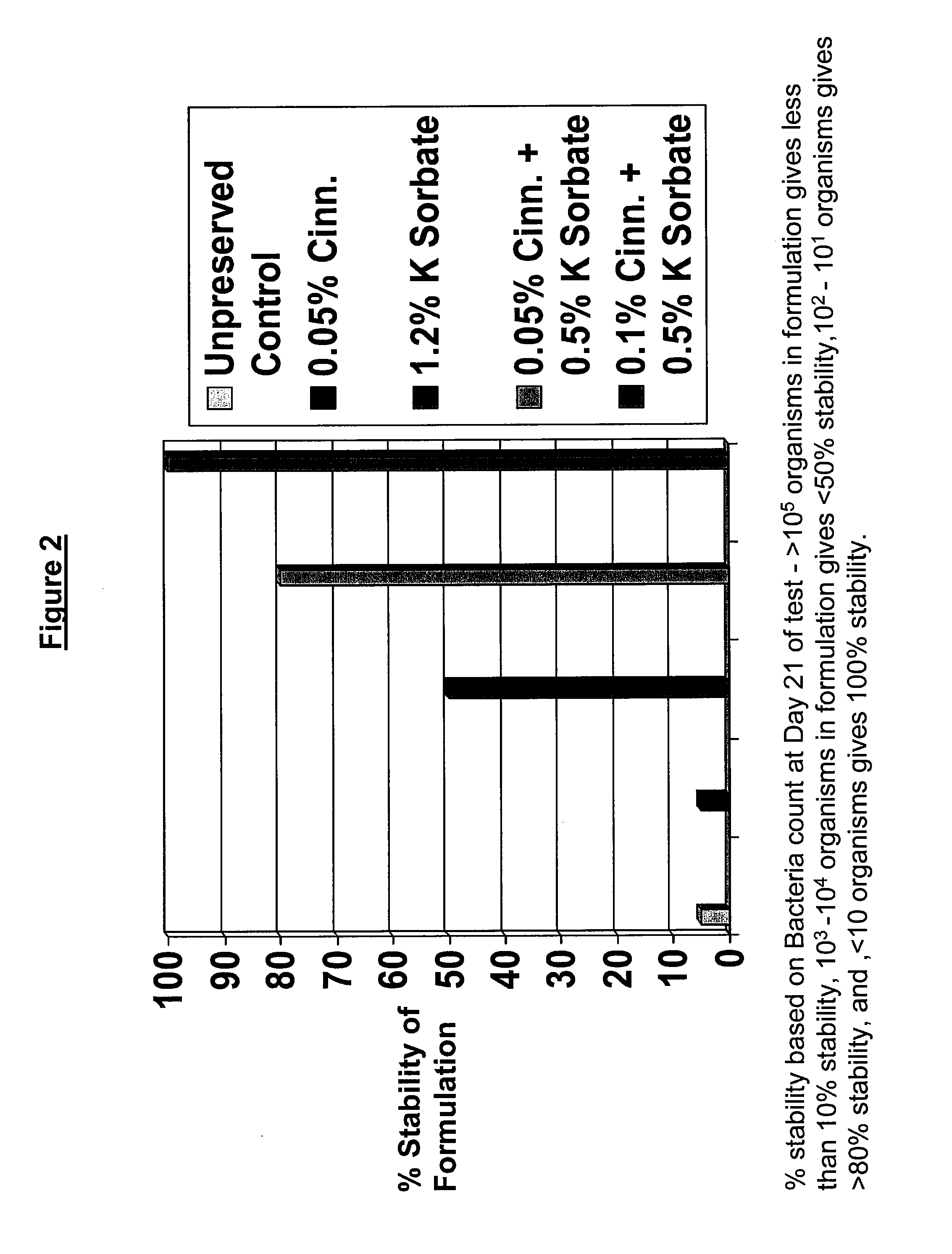
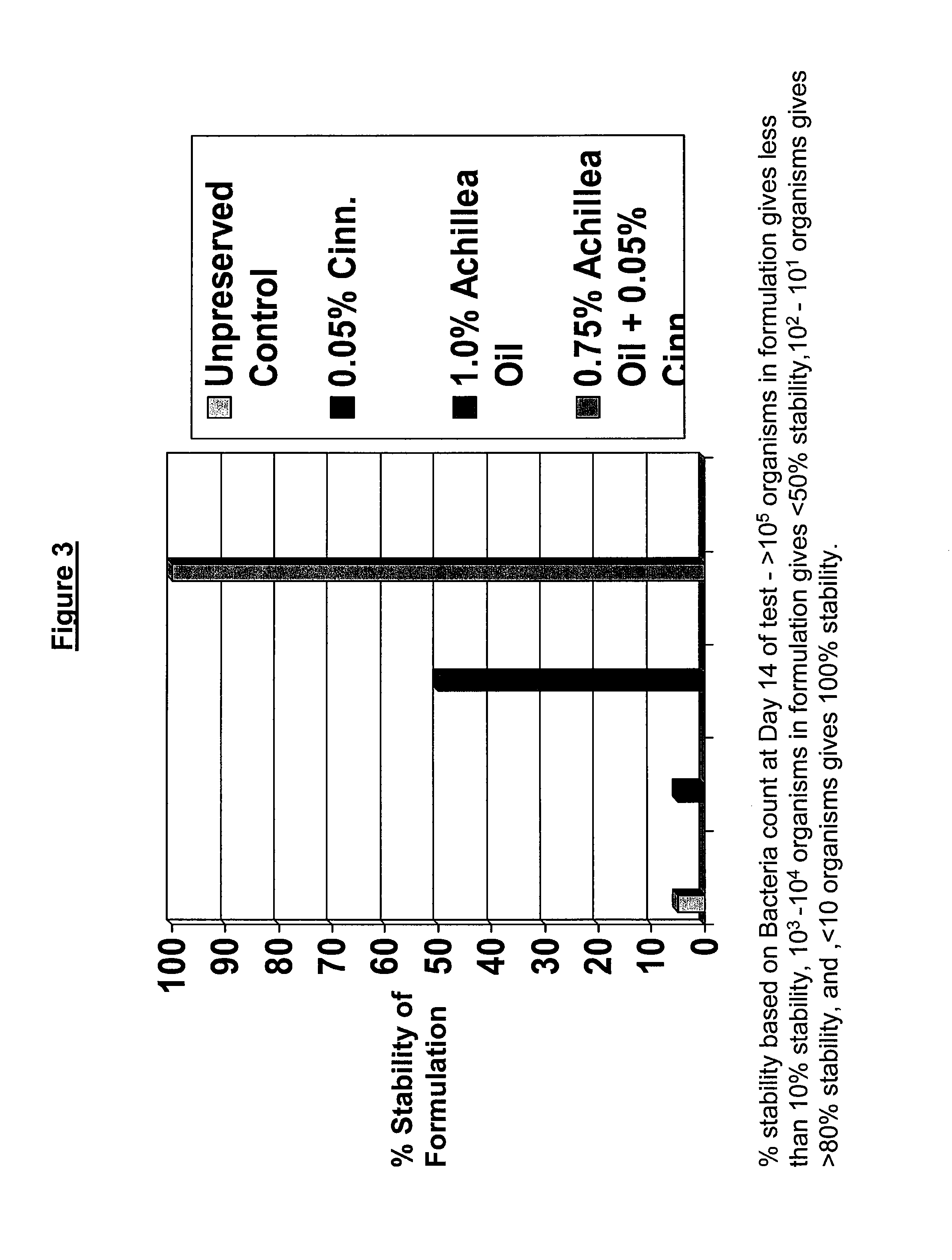
Antimicrobial compositions
The present invention provides an antimicrobial composition comprising an antimicrobial effective amount (such as a preservative, bactericidal, and / or fungicidal effective amount) of a mixture comprising at least two of:(a) lemon grass oil;(b) cinnamaldehyde, cinnamon oil, Cinnamomum cassia, cinnamon extract, cassia leaf oil, 3,4-dihydroxycinnamic acid or salt thereof, or a mixture thereof;(c) sorbic acid, or a salt thereof;(d) erythorbic acid, or a salt thereof;(e) benzoic acid, or a salt thereof;(f) arabinogalactan, galactoarabinan, or a mixture thereof;(g) a hexahydro-iso-alpha-acid, tetrahydro-iso-alpha-acid, or a mixture thereof;(h) Achillea fragrantissima (Santolina fragrantissima Forssk., lavender cotton) oil; and(i) δ-gluconolactone.The present invention also provides a product (preferably a product other than a foodstuff, pharmaceutical, or cosmetic) comprising a preservative effective amount of cinnamaldehyde or a mixture of cinnamaldehyde and one or more alkanol-dialkyl hydantoins.
Owner:LONZA INC
Processing method of bean curd mixed with Premna microphylla Turcz.leaves
InactiveCN101455301AGrass freeNo bitternessCream substitutesFood preparationFlavorPremna microphylla
A processing method of Doufuchai leaves tofu includes: using 85-100 DEG C steam or hot air for de-enzyming 5-20 minutes after cleaning the Doufuchai leaves, and then adding 4 to 6 times of water for homogenate, modulating pH value of the separated slurry to 5.5-6.7, and then adding 0.1-0.2% of gel and 0.01-0.03% of gluconic acid lactone, mixing evenly and then standing to get the Doufuchai leaves tofu. The tofu processed by the method has green or light green appearance, non herbal flavor, no bitter taste, good taste, so that it is, worthy of the name, pure natural green food.
Owner:徐云 +1
Compositions comprising 2-hydroxycarboxylic acids and related compounds, and methods for alleviating signs of dermatological aging
InactiveUS6384079B1Deepening of skin linesSkin lossBiocideCosmetic preparations3-Hydroxypropionic acidPropanoic acid
Uses of topical compositions comprising a 2-hydroxycarboxylic acid or related compound to alleviate or improve signs of skin, nail and hair changes associated with intrinsic or extrinsic aging are disclosed. 2-Hydroxycarboxylic acids and their related compounds include, for example, 2-hydroxyethanoic acid, hydroxypropanoic acid, 2-methyl 2-hydroxypropanoic acid, 2-phenyl 2-hydroxyethanoic acid, 2-phenyl 2-methyl 2-hydroxyethanoic acid, 2-phenyl 3-hydroxypropanoic acid, 2,2-diphenyl 2-hydroxyethanoic acid, 2-hydroxybutane-1,4-dioic acid, 2,3-hihydroxybutane-1,4-dioic acid, 2-carboxy 2-hydroxypentane-1,5-dioic acid, 2-ketopropanoic acid, methyl 2-ketopropanoate, ethyl 2-ketopropanoate, and gluconolactone. Topical application of compositions comprising 2-hydroxycarboxylic acid and / or related compounds has been found to alleviate or improve skin lines; blotches; blemishes; nodules; wrinkles; pigmented spots; atrophy; precancerous lesions; elastotic changes characterized by leathery, coarse, rough, dry and yellowish skin; and other skin changes associated with intrinsic aging or skin damages caused by extrinsic factors such as sunlight, radiations, air pollution, wind, cold, dampness, heat, chemicals, smoke and cigarette smoking. Topical applications of such compositions have also been found to improve the overall qualities of nail and hair affected by intrinsic aging or damaged by extrinsic factors.
Owner:TRISTRATA TECH
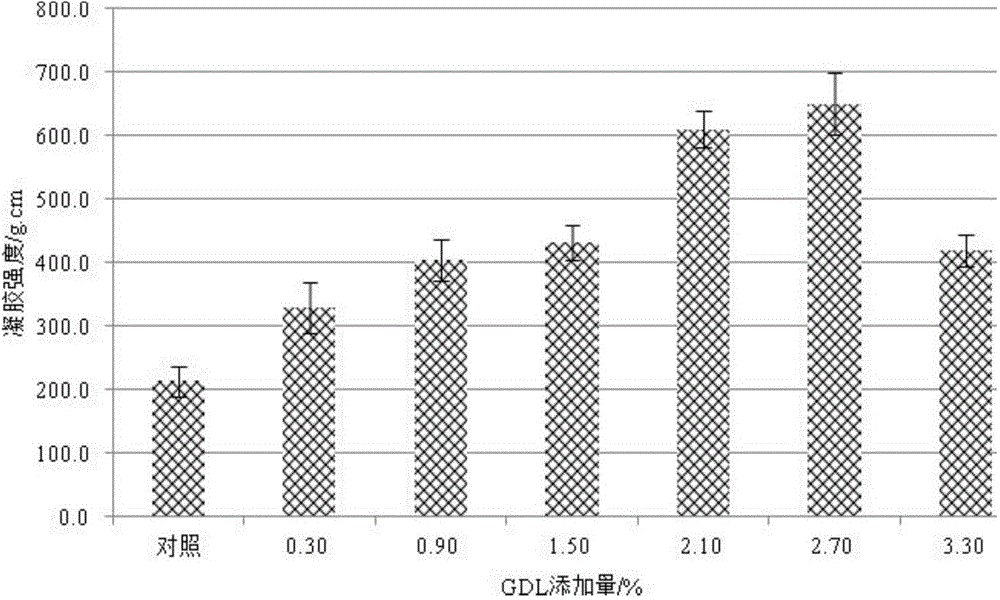
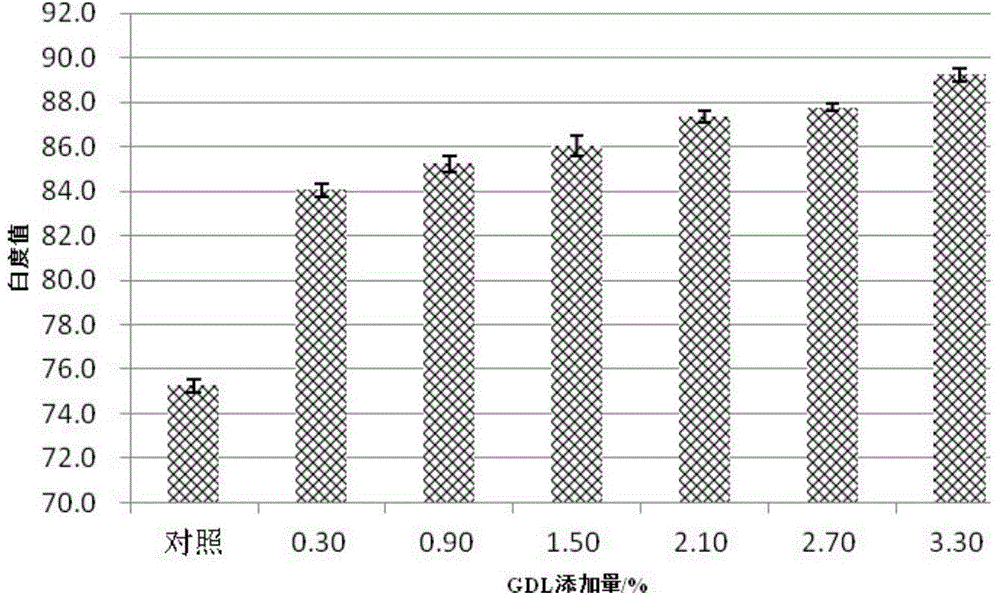
Low-temperature acid-induced minced fish product with high gel strength and production method of minced fish product
ActiveCN104382097AHigh strengthIncrease elasticityFood preparationUltimate tensile strengthFish products
The invention relates to a low-temperature acid-induced minced fish product with high gel strength and a production method of the minced fish product. The production method comprises the steps of unfreezing, chopping via air, chopping via salt, chopping mixture and forming. The gel is induced by acid by slowing hydrolyzing glucolactone and acting on the minced fish protein; compared with the gel strength of the conventional hot gel, the gel strength is improved by over 140%; the texture structure is relatively fine and smooth; the elasticity is improved by over 10%; the whiteness is increased by over 12%; the low-temperature acid-induced minced fish product with high gel strength is a novel instant refrigerated low-temperature acid-induced minced fish product.
Owner:FUJIAN ANJOY FOODS CO LTD +4
Production method of glucolactone
Owner:厦门世达膜环保技术有限公司
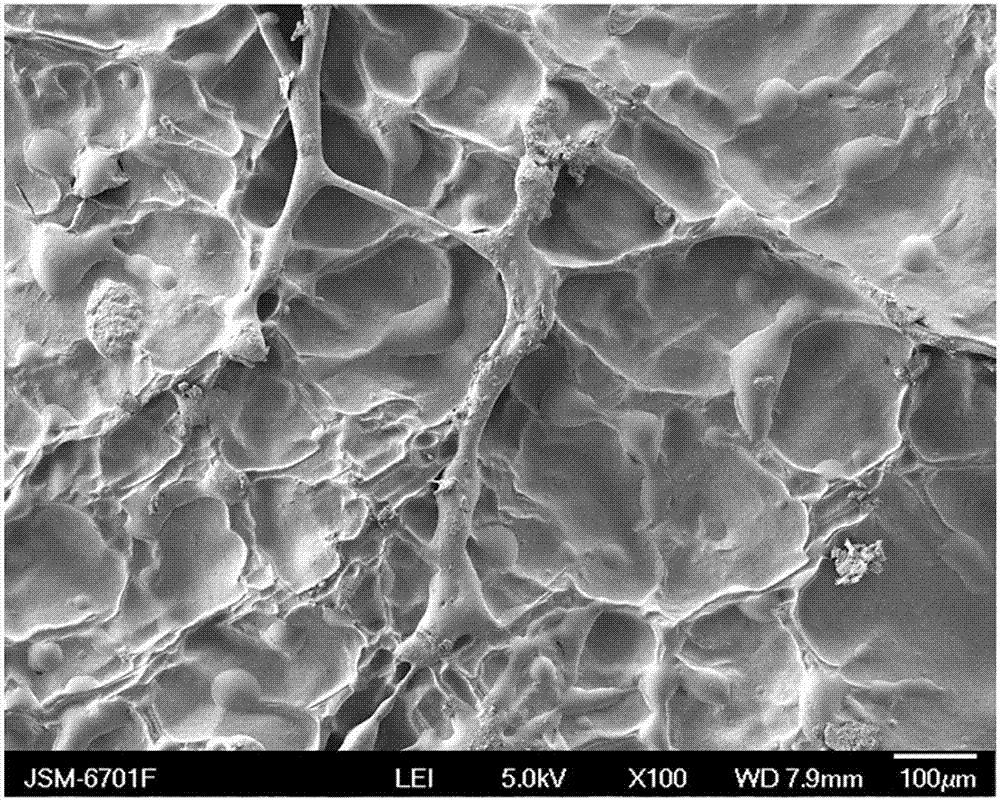
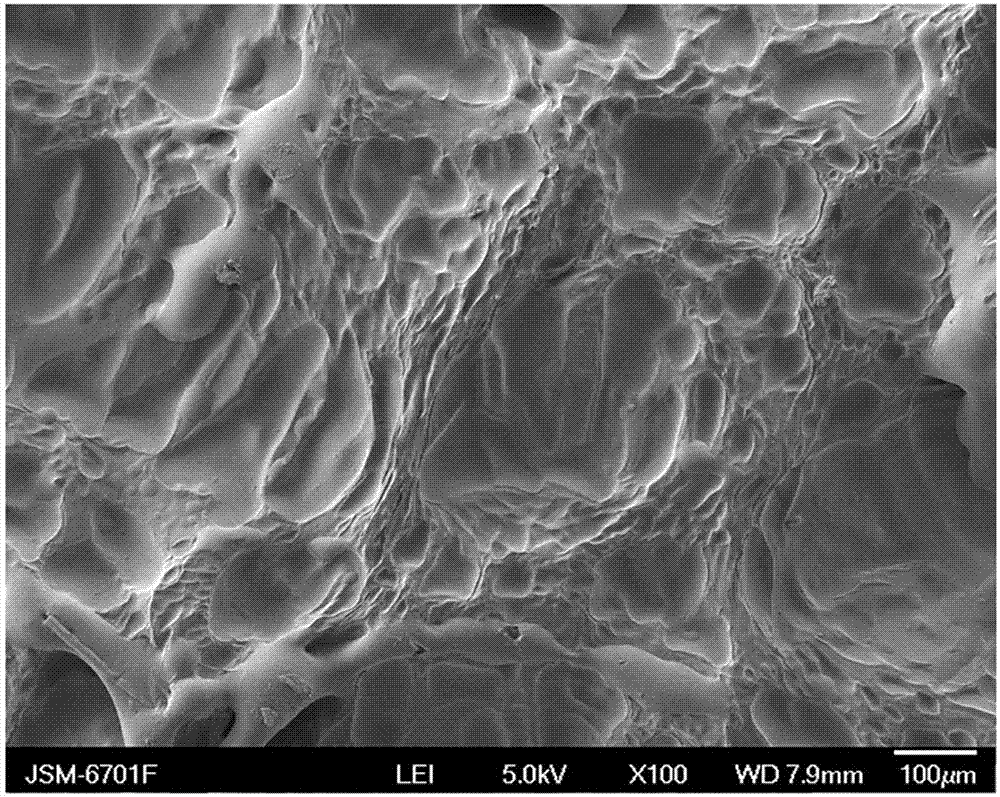
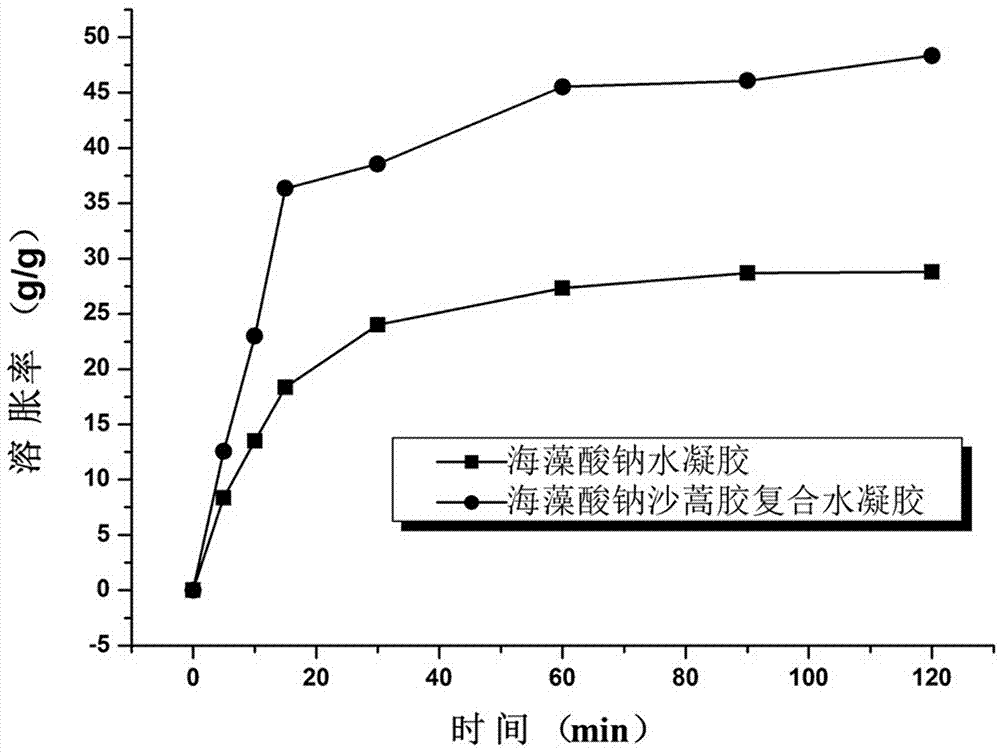
Method for preparing composite hydrogel of sodium alginate and artemisia desertorum seed gum
ActiveCN103087334AImprove water absorptionImprove mechanical propertiesCalcium/strontium/barium carbonatesBiotechnologyOrganic chemistry
The invention discloses a method for preparing a composite hydrogel of sodium alginate and artemisia desertorum seed gum. The method comprises the steps of: introducing the artemisia desertorum seed gum in a sodium alginate solution system, and slowly releasing Ca<2+> in a glucolactone solution by utilizing micropore calcium carbonate, thereby forming the composite hydrogel of sodium alginate and artemisia desertorum seed gum in situ. The properties of the sodium alginate and the artemisia desertorum seed gum are complementary, so that the water absorption capability and the mechanical properties of the sodium alginate base composite hydrogel are enhanced and the application range of the sodium alginate serving as a medicament carrier and a tissue engineering material is expanded. The strength and the gelation time of the the composite hydrogel prepared by the method can be regulated and optimized by changing the proportion of each component and the concentration of glucolactone so as to meet the requirements in different fields. In addition, the method is simple without needing special equipment; the reaction occurs in normal temperature and normal pressure and is simple and controllable; and the method is low in cost and suitable to popularization and application.
Owner:NORTHWEST NORMAL UNIVERSITY
Polyglycol modified trisilicate containing glucose amide group and preparation method
A polyethanediol modified trisiloxane containing glucosylamido used as the assistant of agricultural chemical to decrease the surface tension of water is prepared through catalytic reaction between hexamethyldisiloxane and aminosilane to obtain aminotrisiloxane, reacting on lactone gluconate in methanol to obtain trisiloxane containing glucosylamido, and modifying it by polyethanediol method glicideether.
Owner:CHINA RES INST OF DAILY CHEM IND
Injected gel type bone repairing biological active material and its preparing method
InactiveCN1586621AGood biocompatibilityEasy to usePeptide/protein ingredientsSkeletal disorderPyrrolidinonesBiocompatibility
Of the bioactive gel injection material for bone repairing, each dose consists of component A in 1 ml and component B in 45-55 mg. The component A contains sodium alginate 10-40 mg, bone morphogenetic protein 0.1-1 mg and mannitol 10-20 mg in each ml of bacteria-free physiological saline. Of the component B, each mg contains water insoluble calcium compound 0.05-0.15 mg, gluconolactone 0.05-0.3 mg and polyvinyl pyrrolidone 0.004-0.016 mg except mannitol. The injection material for bone repairing has excellent biocompatibility and simple and safe use, and may be injected to required bone treating position. Animal experiment shows that it has similar bone forming activity to operation implanted solid bone repairing material containing bone morphogenetic protein.
Owner:徐放
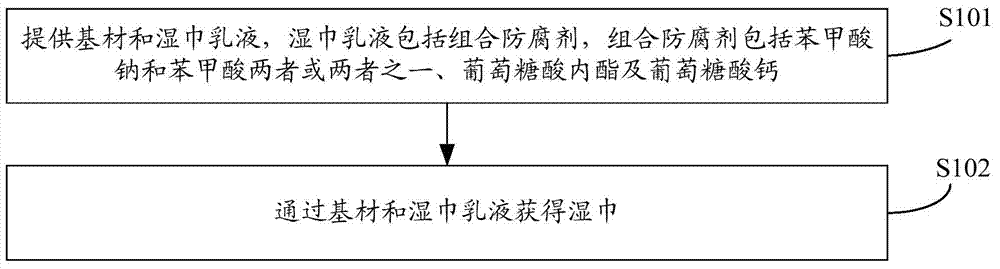
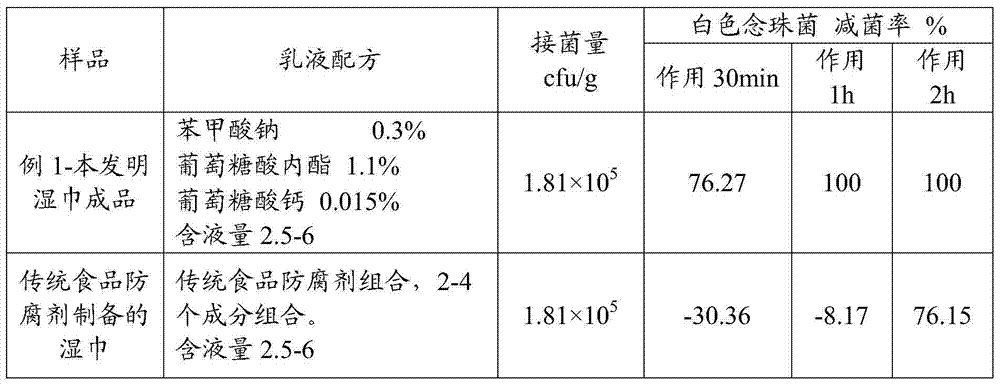
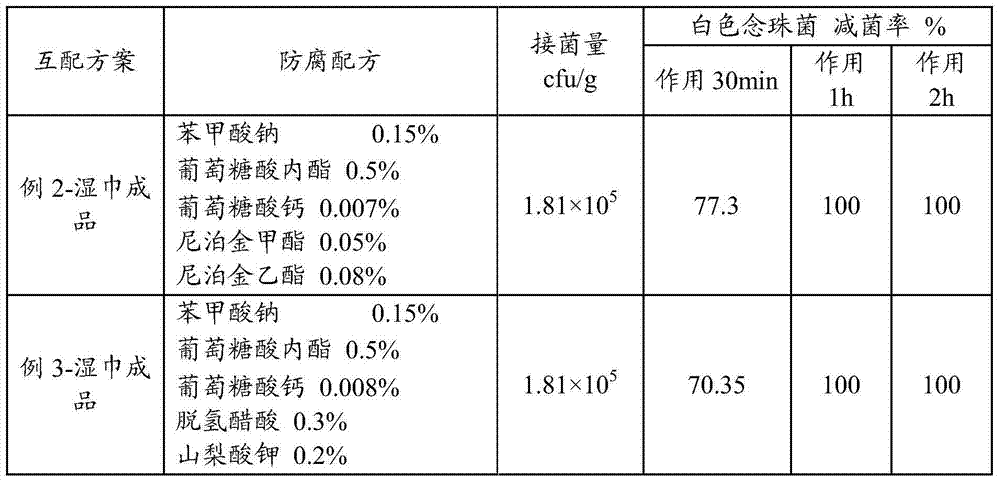
Wet tissue emulsion, wet tissue and preparation method of wet tissue
InactiveCN103919689AImprove stabilityImprove securityBiocideCosmetic preparationsBenzoic acidEmulsion
The invention discloses a wet tissue emulsion, a wet tissue and a preparation method of the wet tissue. The method comprises a step of providing a base material and the wet tissue emulsion, the wet tissue emulsion comprises a composition antiseptic, the composition antiseptic comprises two or one of sodium benzoate and benzoic acid, glucolactone and calcium gluconate; and the wet tissue is obtained by the base material and the wet tissue emulsion. Through the above mode, the wet tissue product has the advantages of high stability, slight smell, long shelf-life, rapid sterilization and high security.
Owner:GOLD HONG YE PAPER
Antimicrobial compositions
The present invention provides an antimicrobial composition comprising an antimicrobial effective amount (such as a preservative, bactericidal, and / or fungicidal effective amount) of a mixture comprising at least two of:(a) lemon grass oil;(b) cinnamaldehyde, cinnamon oil, Cinnamomum cassia, cinnamon extract, cassia leaf oil, 3,4-dihydroxycinnamic acid or salt thereof, or a mixture thereof;(c) sorbic acid, or a salt thereof;(d) erythorbic acid, or a salt thereof;(e) benzoic acid, or a salt thereof;(f) arabinogalactan, galactoarabinan, or a mixture thereof;(g) a hexahydro-iso-alpha-acid, tetrahydro-iso-alpha-acid, or a mixture thereof;(h) Achillea fragrantissima (Santolina fragrantissima Forssk., lavender cotton) oil; and(i) δ-gluconolactone.The present invention also provides a product (preferably a product other than a foodstuff, pharmaceutical, or cosmetic) comprising a preservative effective amount of cinnamaldehyde or a mixture of cinnamaldehyde and one or more alkanol-dialkyl hydantoins.
Owner:ARXADA LLC
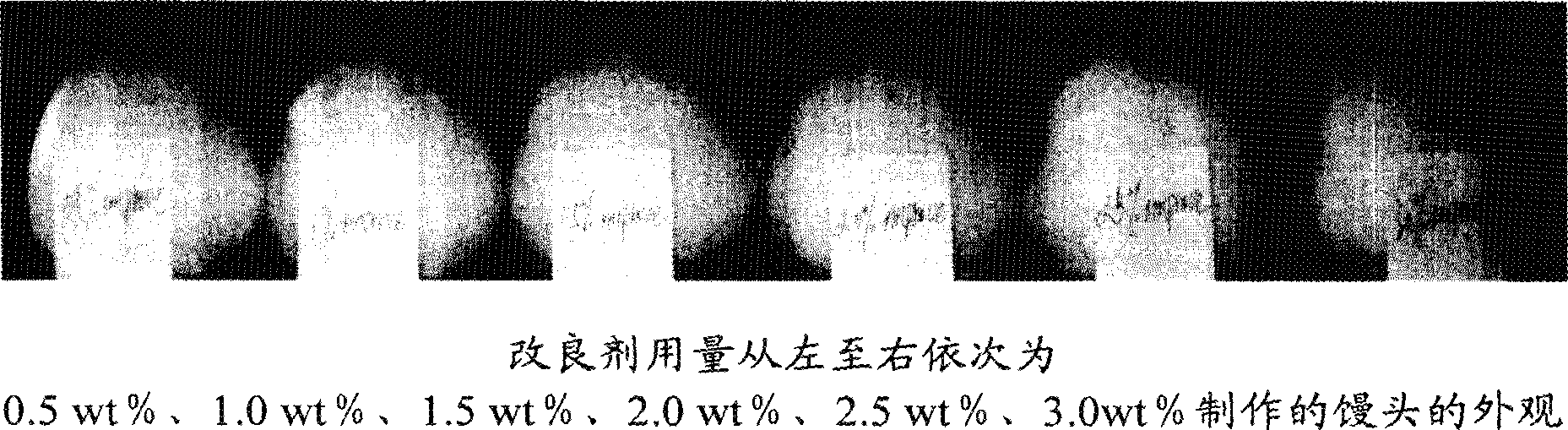
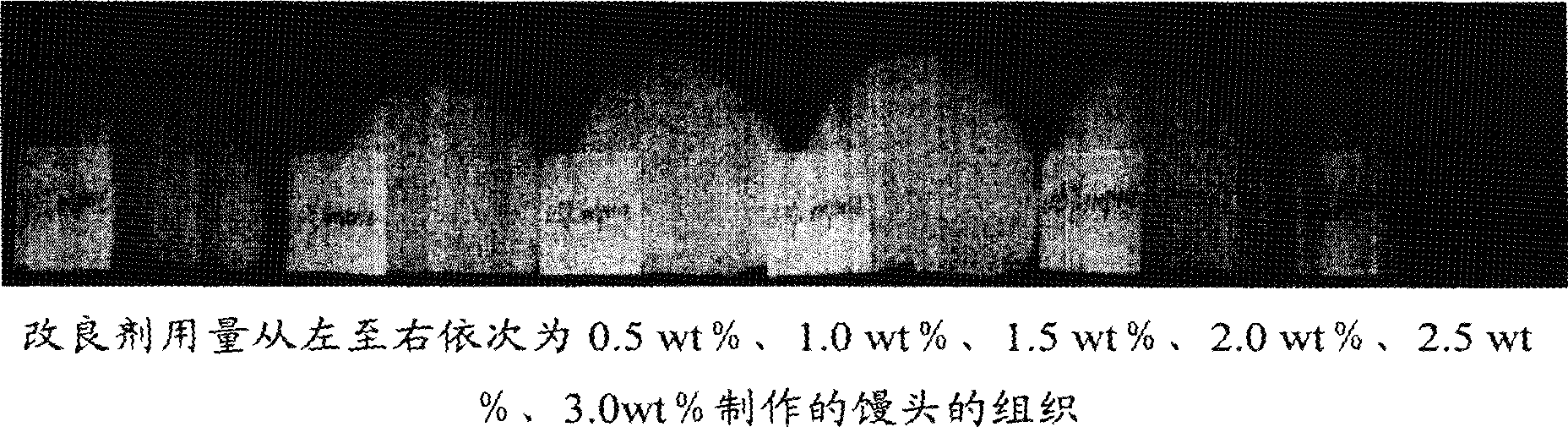
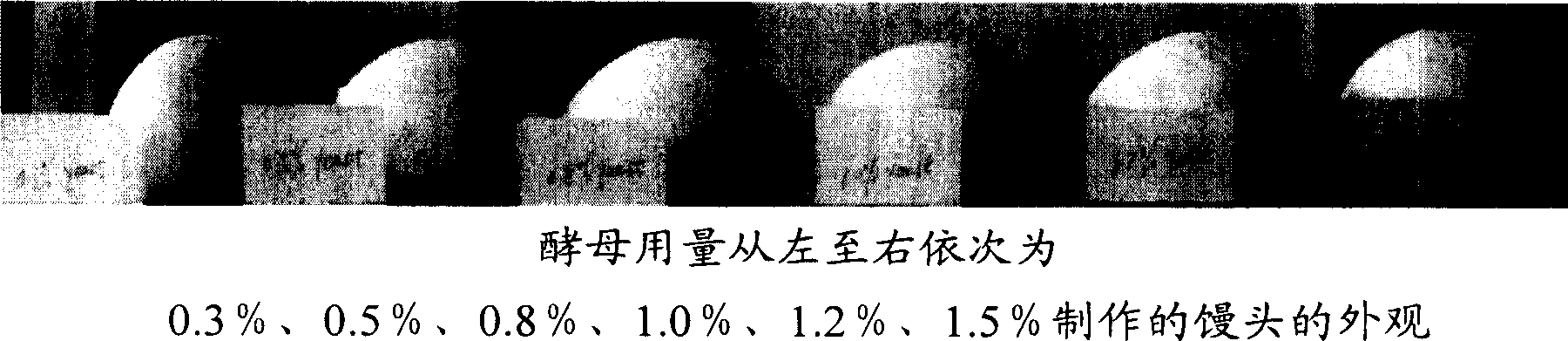
Improver of self-rising flour, self-rising flour using the improver and steamed food
ActiveCN101396036AImprove performanceImprove qualityDough treatmentPre-baking dough treatmentSodium bicarbonateVitamin C
The invention relates to a self-raising flour modifier and a self-raising flour which uses the modifier, in particular to a self-raising flour modifier, which comprises (on the basis of total weight of the self-raising flour modifier) 28-40% of sodium bicarbonate, 4-7% of disodium dihydrogen pyrophosphate, 4-6% of calcium hydrogen phosphate, 24-28% of calcium biphosphate, 10-20% of glucolactone, 0.01-0.03% of fungal-Alpha-amylase, 0.01-0.02% of glucose oxidase, 0.05-0.10% of xylanase, 0.3-0.45% of vitamin C, 0.5-2% of calcium peroxide, 3-5% of CSL-SSL, 2-6% of gluten and residual quantity of maize starch. The self-raising flour modifier can effectively increase the volume of steamed food such as Chinese steamed bread, steamed and stuffed bun and the like which are made of the self-raising flour, leads the food to have smooth surface, uniform and fine tissue, more delicious taste and no bad flavor. The invention also relates to the self-raising flour and the steaming food which contain the modifier.
Owner:ANGELYEAST CO LTD
Calcium alginate gel bead with shell of porous calcium carbonate microsphere and its preparing method
InactiveCN1985995AImprove adsorption capacityImprove surface strengthInorganic non-active ingredientsGranular deliveryOil emulsionMicrosphere
The present invention relates to preparation process of calcium alginate gel bead with shell of porous calcium carbonate microsphere. Water phase of sodium alginate is dispersed in oil phase containing porous calcium carbonate microsphere and the mixture is emulsified to form water-in-oil emulsion. The porous calcium carbonate microsphere is adsorbed to the surface of the emulsion drop to form the shell stabilizing the emulsion drop, and water solution of gluconolactone is added for the geltinization reaction of sodium alginate. The present invention makes it possible to match prepare micro bead comprising porous calcium carbonate microsphere as shell and calcium alginate gel as core.
Owner:SOUTH CHINA UNIV OF TECH
Polylactic-co-glycolic acid (PLGA)/calcium carbonate compound microsphere with porous shell and preparation method for compound microsphere
ActiveCN102772825AEasy to prepareUniform shapeMicroballoon preparationProsthesisFreeze-dryingMethylene Dichloride
The invention discloses a polylactic-co-glycolic acid (PLGA) / calcium carbonate compound microsphere with a porous shell and a preparation method for the compound microsphere. The preparation method comprises the following steps of: a, dissolving PLGA in a methylene dichloride organic solvent to obtain a PLGA oil phase; adding calcium carbonate powder to the PLGA oil phase; stirring and performing ultrasonic treatment to obtain organic / inorganic uniform mixed liquid; b, dissolving glucolactone in polyvinyl alcohol (PVA) aqueous solution to obtain acid PVA aqueous solution; c, dispersing the mixed liquid obtained in the step a into the acid PVA aqueous solution obtained in the step b under the condition of stirring to obtain oil-in-water single emulsion; d, continue stirring under the condition of reduced pressure to enable methylene dichloride in an oil phase drop to volatilize to obtain solidified compound microspheres; and e, collecting the microspheres in the step d; washing by using deionized water; and performing freeze drying. A pore-forming agent is not introduced by the method disclosed by the invention, thus, a porous structure exists on the surface of the prepared compound microsphere, and the compound microsphere is in favor of cell growth.
Owner:SOUTH CHINA UNIV OF TECH
Slow release bulk blend fertilizer and preparation method thereof
InactiveCN102924139AImprove absorption rateImprove fertilityBio-organic fraction processingOrganic fertiliser preparationDispersityAbsorption rate
The invention discloses a slow release bulk blend fertilizer and a preparation method thereof. The slow release bulk blend fertilizer comprises a composition A and a composition B, wherein the composition A contains urea, cellulose, tea seed cake, carbon black, polypropylene glycol, diammonium phosphate, triple superphosphate, potassium sulphate, potassium chloride, modified plant ash, sweet potato leaves, mushroom dreg, distillers' grains, water soluble phenol-formaldehyde resin, zinc sulfate, ferrous sulfate and nitrogen-fixing bacteria; and the composition B contains pond sludge, acrylic emulsion, ethyl acetate, ammonium acrylate, amino acid, calcium powder, alpha-pimacol, glucolactone, ammonium molybdate, micronutrients fertilizer, nano-montmorillonite and silicate potash fertilizer. Plant straws are incinerated and modified by using epoxidized soybean oil and calcium lignosulphonate, thus an absorption structure is increased, blocking is prevented and dispersity is increased; and resistance to lodging, diseases and drought of plants is effectively provided, the plant absorption rate is high, the damage to soil is correspondingly reduced, waste materials are recycled, and the fertility of a fertilizer is effectively increased.
Owner:ANHUI SUNSON CHEM
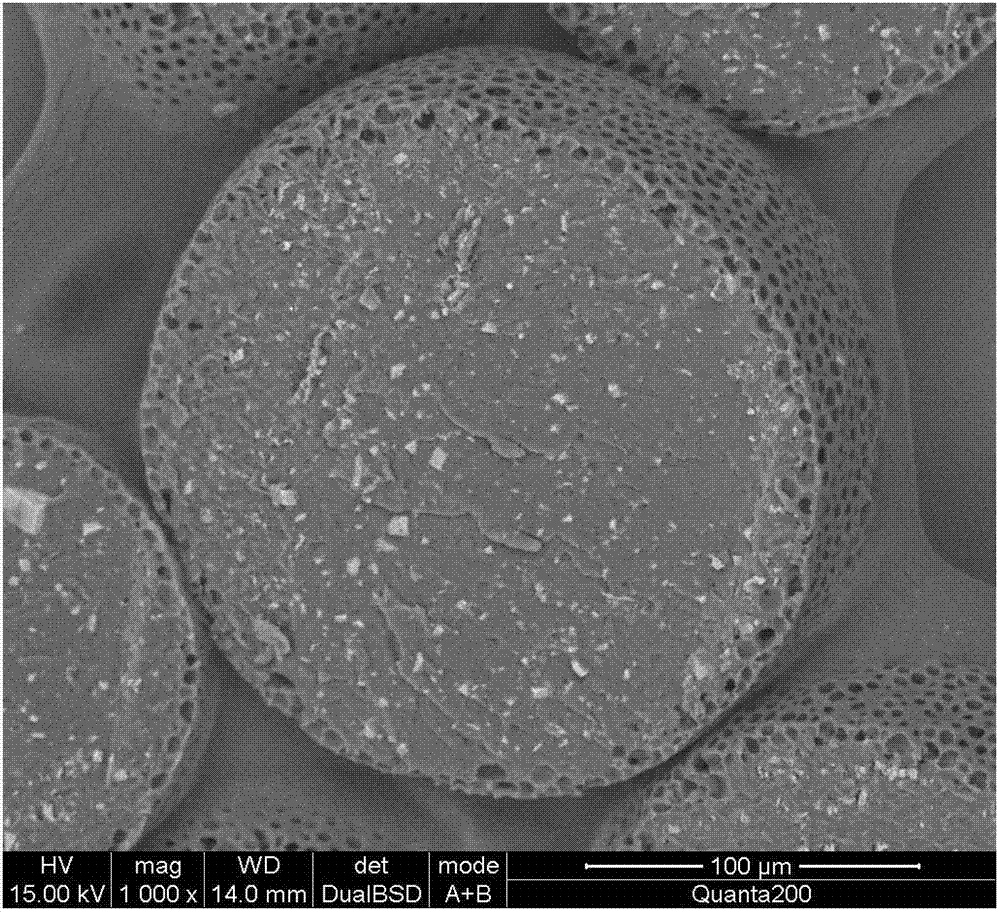
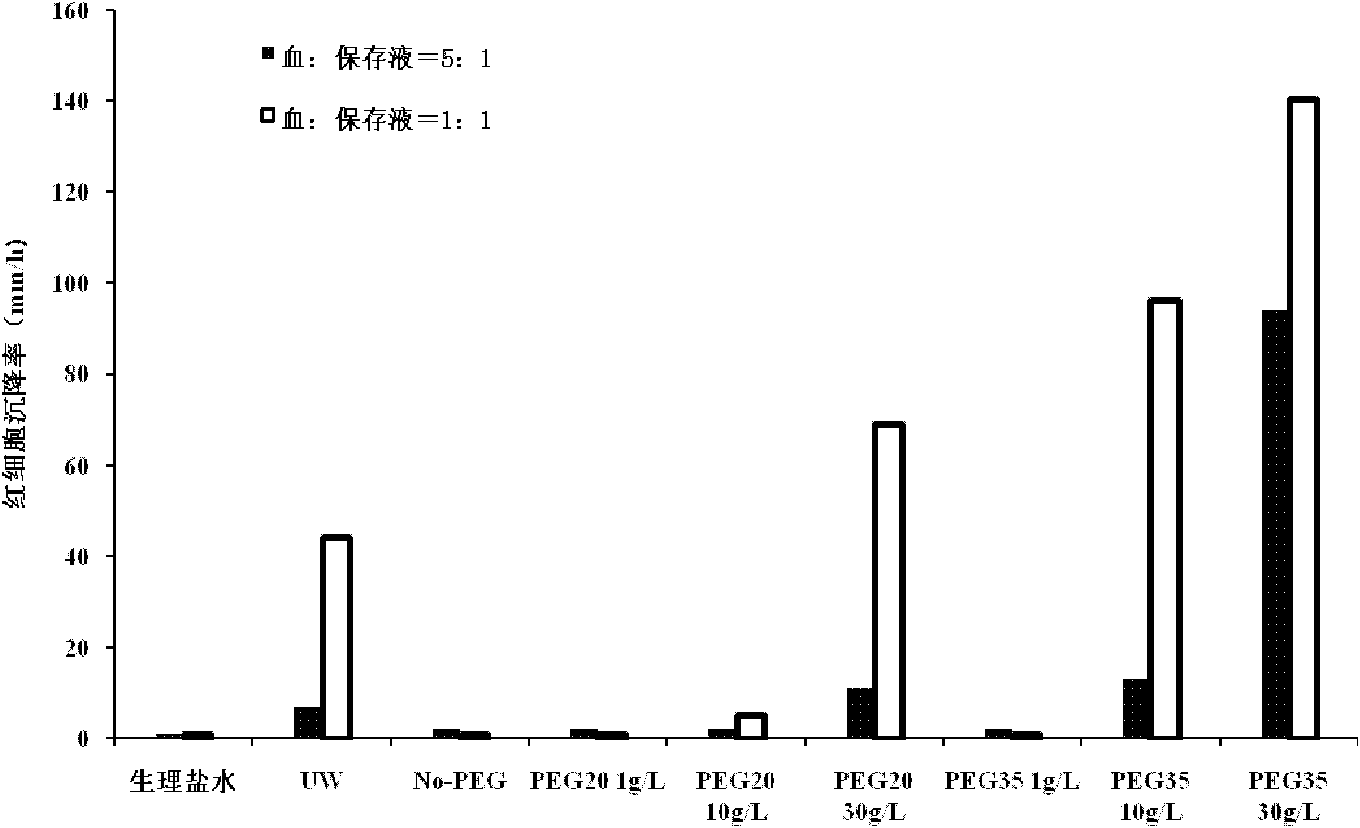
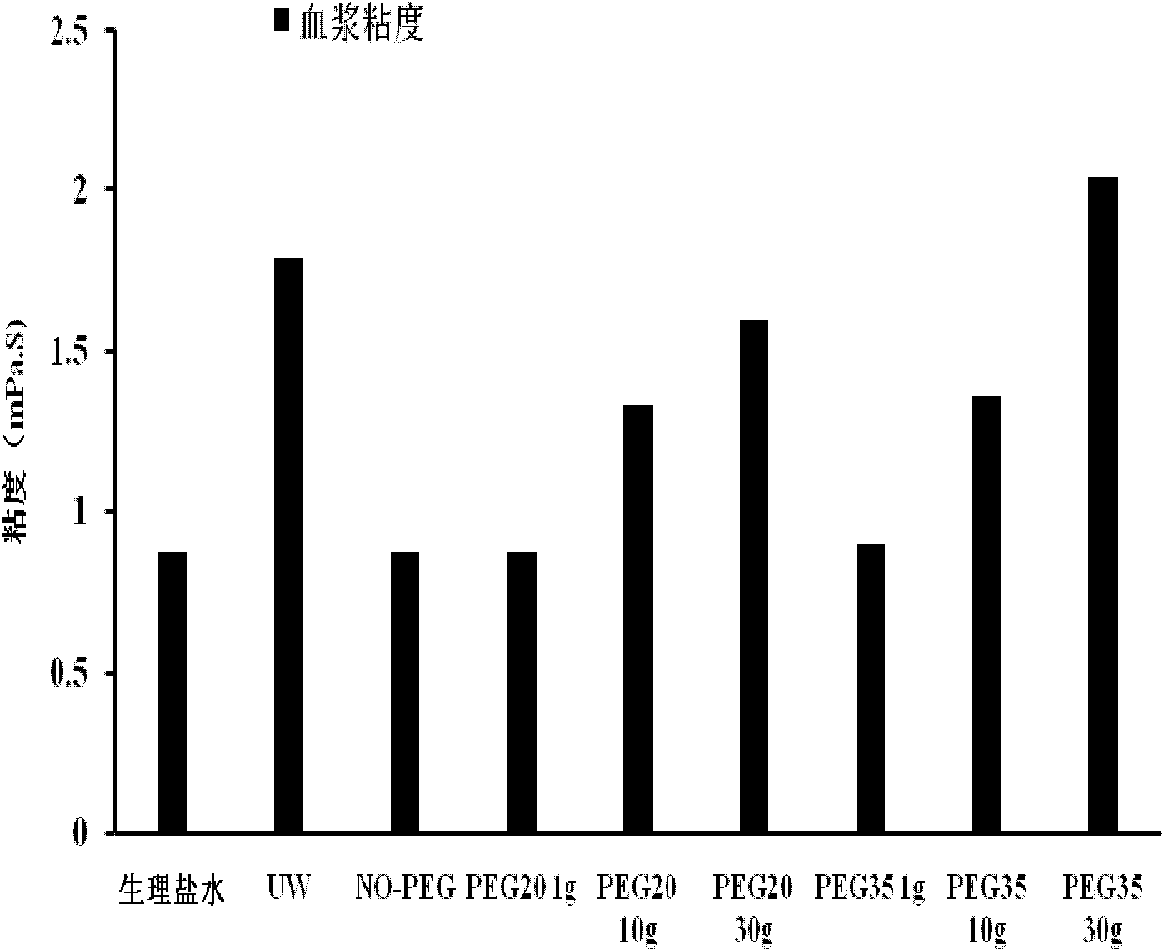
Organ preservation solution and method for preparing same
ActiveCN102726366AAvoid damageDisadvantages of Avoiding PrecipitationDead animal preservationPhosphateArginine
Owner:SHANGHAI GENEXT MEDICAL TECH
Double-layer composite hydrogel for repairing bone and cartilage tissues as well as preparation method and application of double-layer composite hydrogel
ActiveCN106267357ARepair joint damageTightly boundTissue regenerationProsthesisBond interfaceMedicine
The invention discloses a double-layer composite hydrogel material applicable to bone and cartilage repair. A bone repair layer consists of a sodium alginate / akermanite / glucolactone composite hydrogel; and a cartilage repair layer consists of a sodium alginate / agarose composite hydrogel. The invention also discloses a preparation method and an application of the double-layer composite hydrogel. The double-layer composite hydrogel disclosed by the invention can simulate bone and cartilage tissues, and interfaces of the double-layer composite hydrogel are closely connected, so that a bone and cartilage bonding interface can be simulated. The double-layer composite hydrogel disclosed by the invention, while promoting the regeneration of the bone and cartilage tissues, can also promote angiogenesis in new bone tissues, so that bone and cartilage tissue repair is promoted.
Owner:SHANGHAI JIAO TONG UNIV
Method of clarifying oily waste water
Owner:ECOLAB USA INC
Preparation method of low-sugar preserved fruits of cherry tomatoes
InactiveCN104719598AMeet the requirements for low-sugar candied fruitConfectionerySweetmeatsChlorideSugar
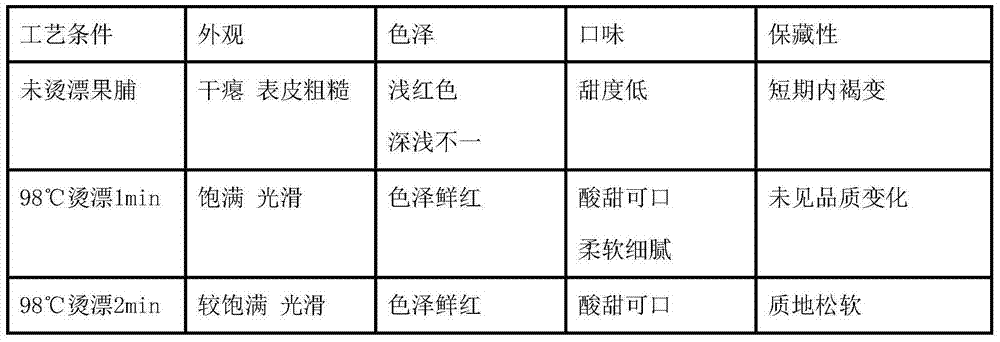
The invention discloses a preparation method of low-sugar preserved fruits of cherry tomatoes. According to the preparation method, the cherry tomatoes are used as raw materials for preparing the low-sugar preserved fruits, and the optimal process conditions are as follows: performing color protection by using 0.1% of NaHSO3, 0.1% of Vc, 0.1% of citric acid and 0.1% of CaCl2 for 1h; blanching for 1min at the temperature of 98 DEG C; hardening by adopting 3% of delta-gluconolactone and 0.1% of calcium chloride for 4h; performing sugar infusion by using 35% of sugar liquid, 15% of dextrin and 0.3% of sodium carboxymethylcellulose for 30min under the vacuum condition that the vacuum degree is 0.07MPa; performing sugar boiling by using 50% of sugar liquid at the temperature being below 80 DEG C for 1h, wherein the temperature is lower than 80 DEG C; and drying with hot air for 17h. The results prove that by synthesizing three major indexes, namely sugar content, appearance and guarantee period, the low-sugar preserved fruits of the cherry tomatoes are better than those reported in literature and are basically in line with the requirements of the low-sugar preserved fruits.
Owner:ZHONGZHOU UNIV
Long-acting cockroach killing gel bait
The invention relates to a long-acting gel bait used for killing cockroaches, which is characterized in that the gel bait consists of fipronil, boric acid, pectin, glucolactone, milk powder, white sugar, honey, soy meal, biscuit powder and balm. The gel bait is strong in allure, convenient in use, safe, highly efficient, low in toxicity, long-acting and environment-friendly and can attract the cockroaches to eat actively and gather more cockroaches to eat the gel bait via the habits of the cockroaches, thus realizing better effect for attracting and killing the cockroaches.
Owner:郑磊
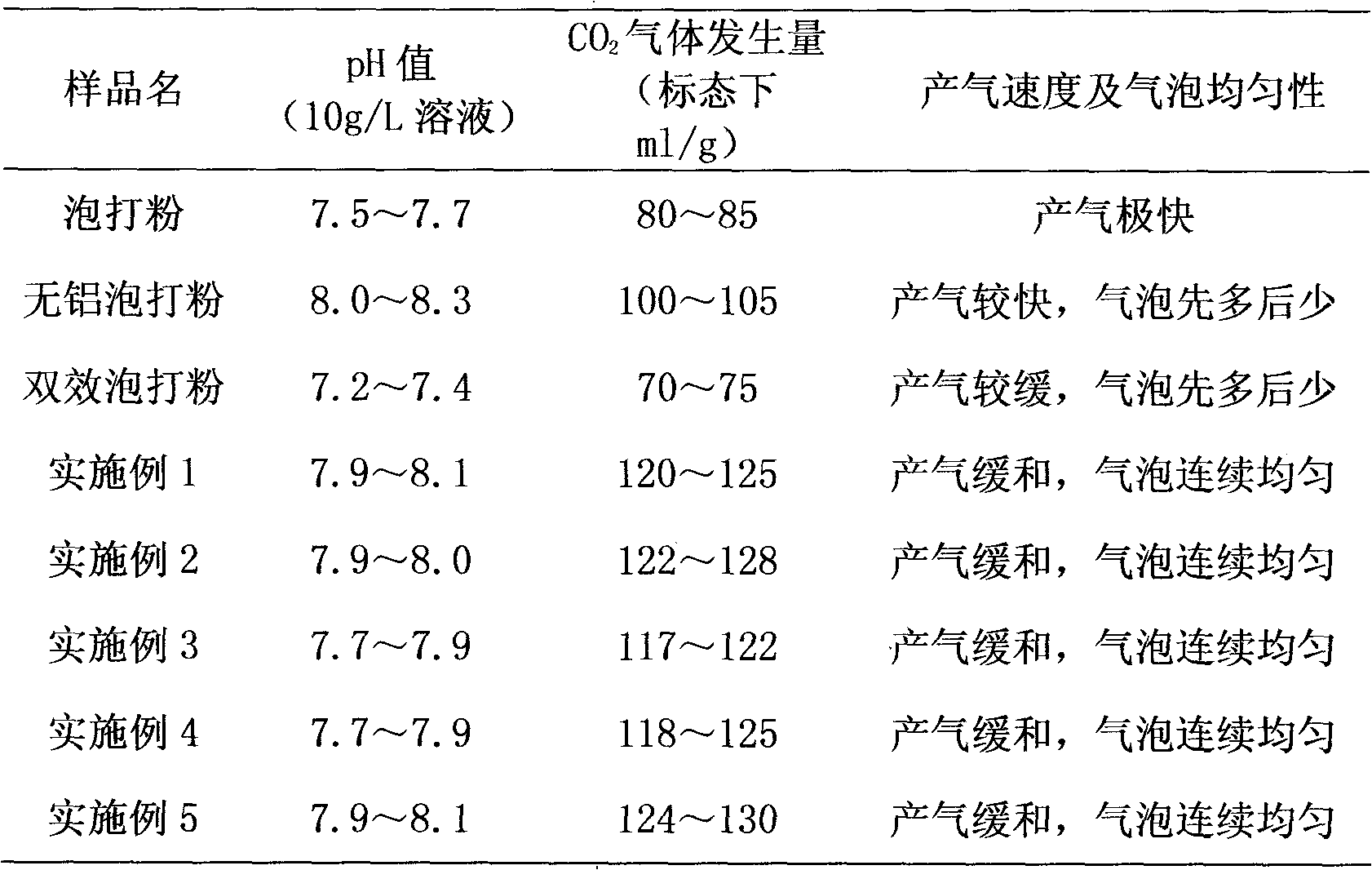
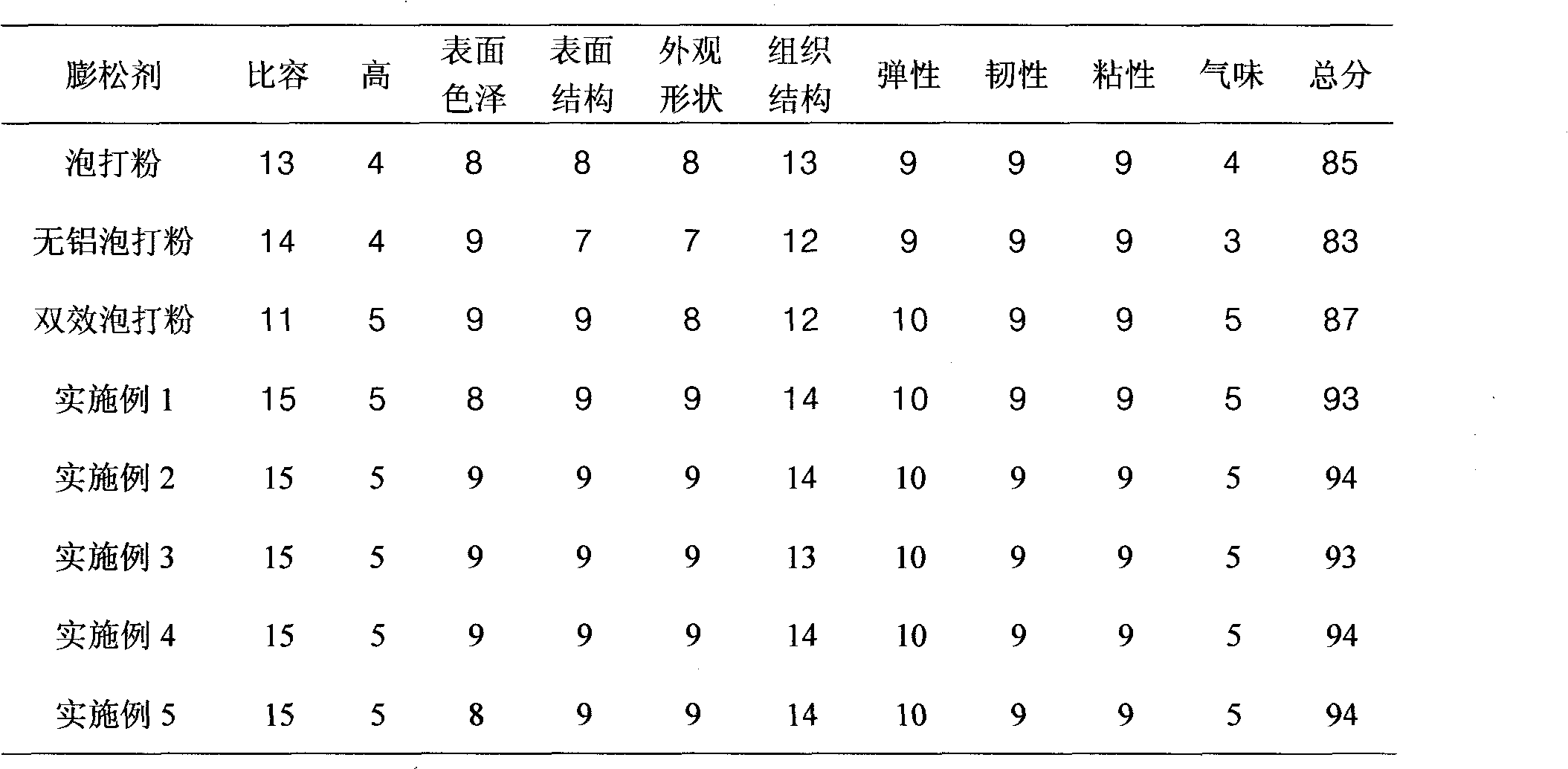
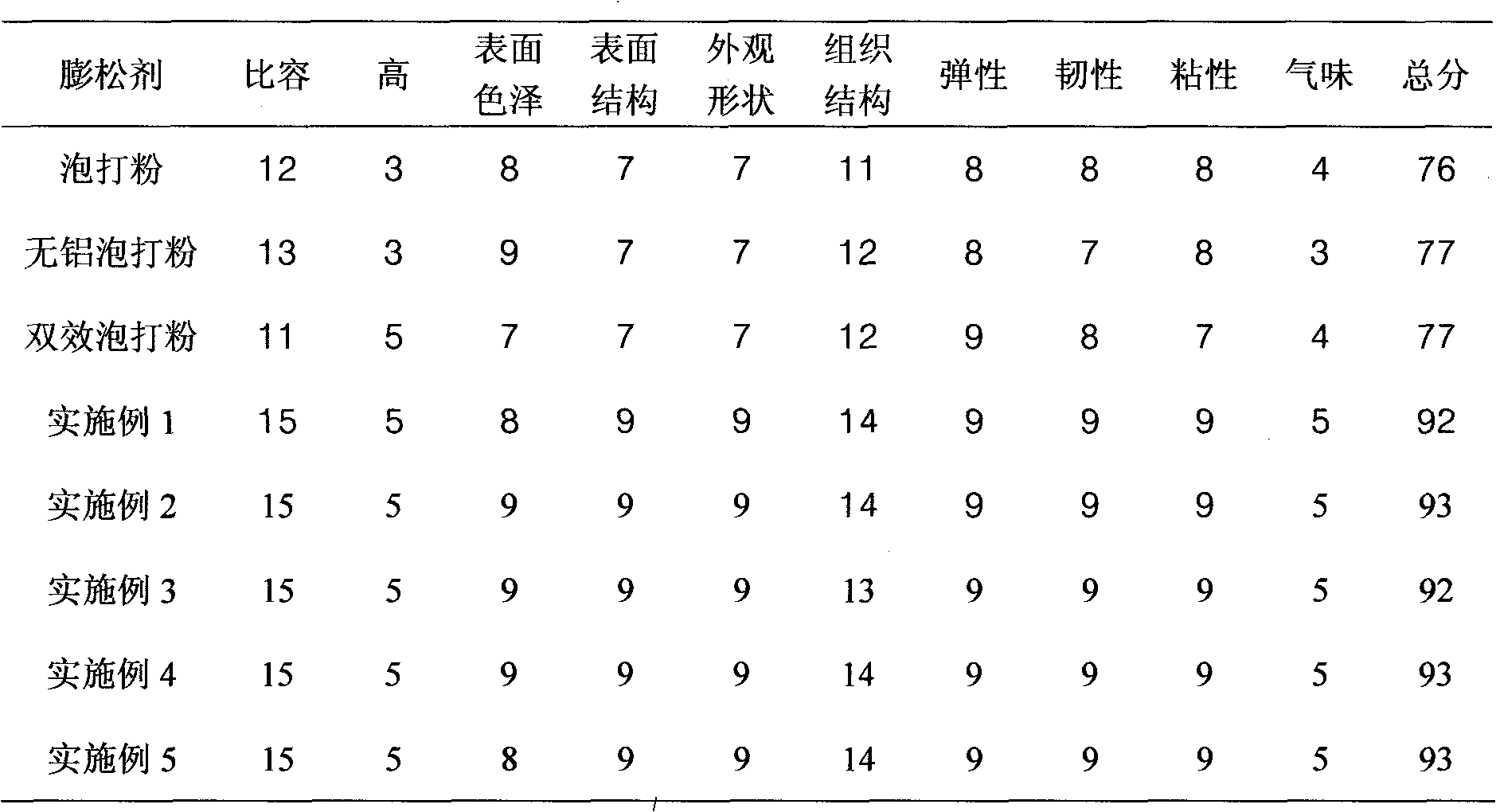
Aluminum-free baking powder
The invention discloses an aluminum-free baking powder, which mainly comprises the following raw materials in percentage by weight: 35-40% of sodium hydrogen carbonate, 18-23% of glucolactone, 10-15% of calcium carbonate, 8-13% of starch, 4-8% of glycerin monostearate, 4-8% of phospholipids, 2-5% of citric acid and 2-5% of tartaric acid, wherein 1-2% in percentage by weight of ascorbyl palmitate is also added in the aluminum-free baking powder. In the invention, the alkalinity of the baking powder is moderate; the gas producing speed is gentle at the same time as a higher total gas productionrate is ensured; bubbles are continuous and uniform; the appearance of the product can be obviously improved in actual application; internal tissues of the product are uniform and fine; and good elasticity is kept; moreover, the aluminum-free baking powder has good functions of water retention and modification, and can ensure that the product also retains the uniform and fine tissue structure, and good elasticity and toughness after being frozen at a low temperature; the aluminum-free baking powder is suitable for frozen rice and flour products, and has good oxidation resistance; and the oxidative rancidity of the product can be obviously inhibited, and the shelf life of the product can be effectively prolonged.
Owner:GUANGZHOU FOOD IND RES INST
Multi-effect moisturizing cream and preparation method thereof
InactiveCN109364008AAnti-blue light hasAnti-wrinkle and whitening effectCosmetic preparationsToilet preparationsWrinkle skinPunica
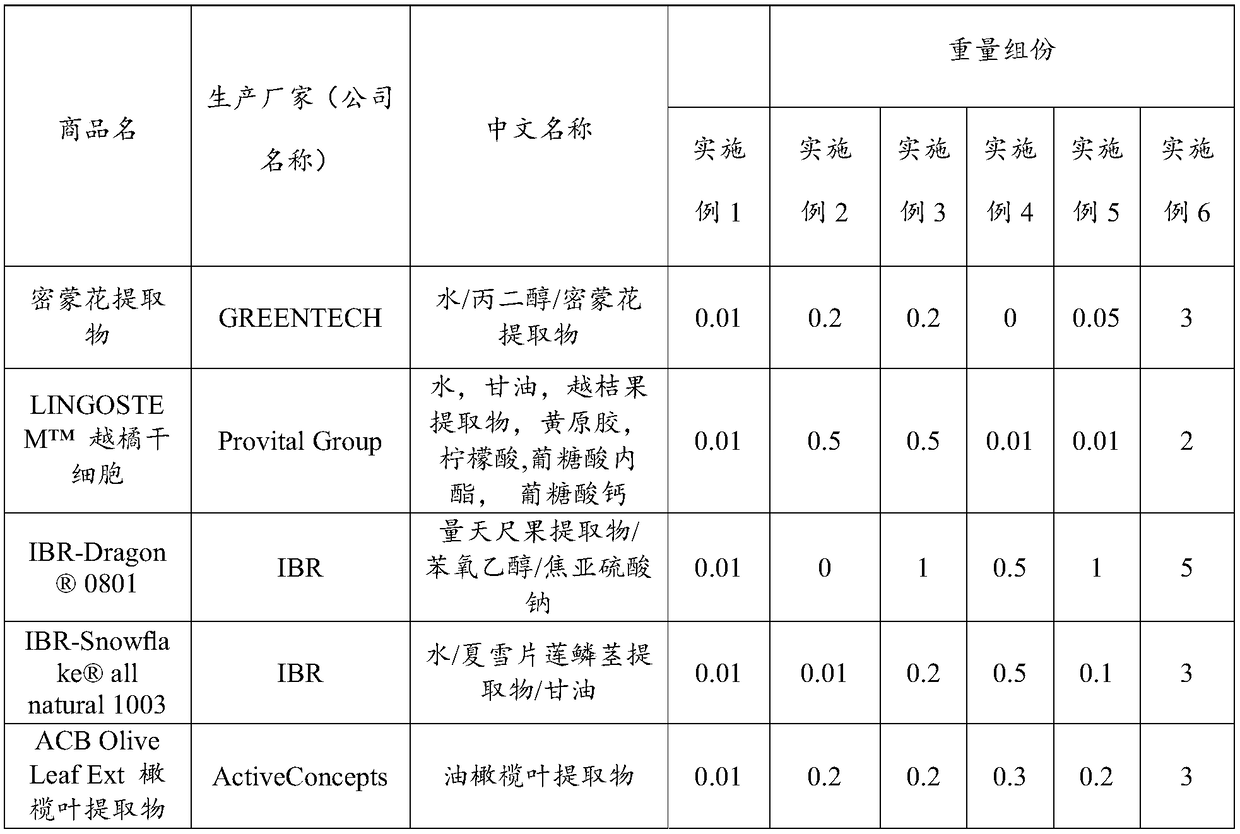
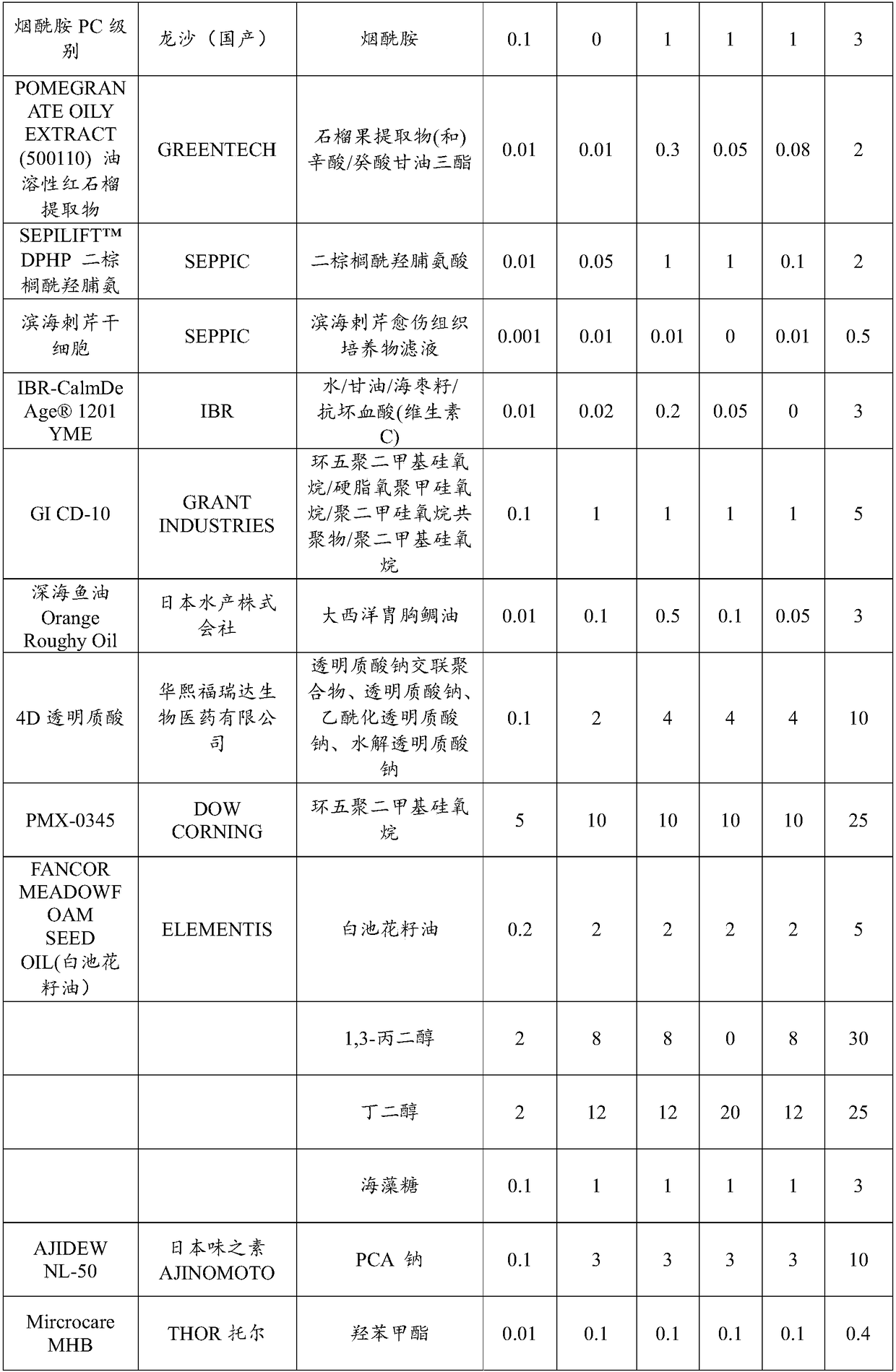
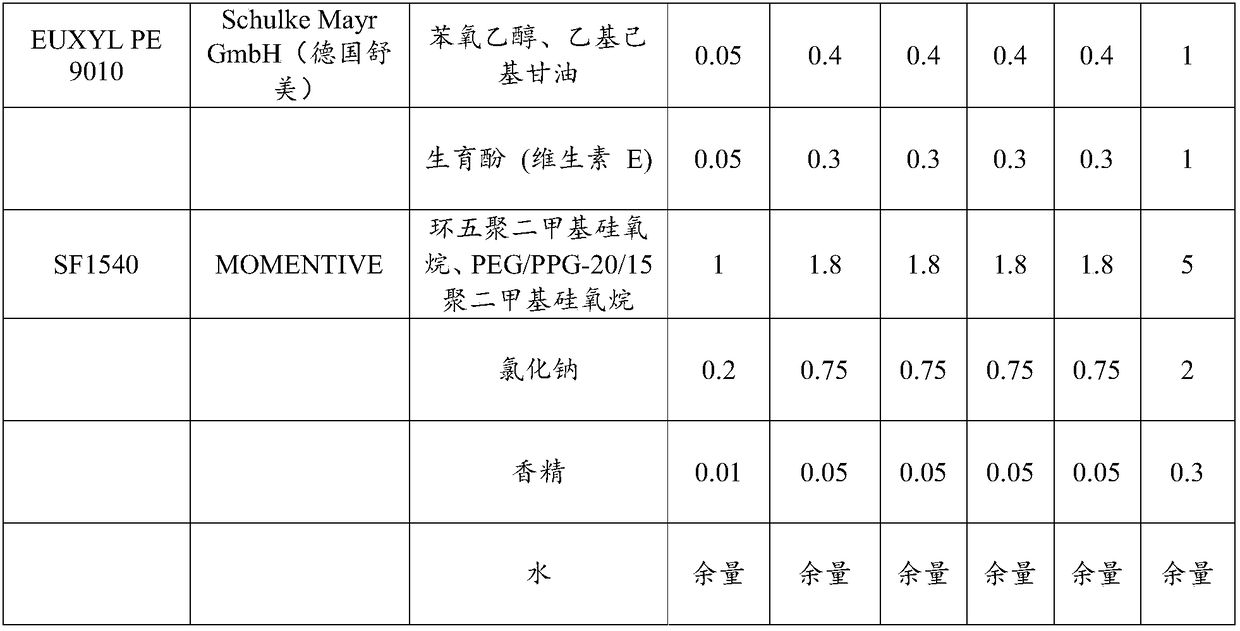
The invention provides multi-effect moisturizing cream. The multi-effect moisturizing cream is prepared from the following components in parts by weight: 0.01 to 3 parts of butterflybush flower extract, 0.01 to 2 parts of vaccinium myrtillus fruit extract, 0.01 to 2 parts of xanthan gum, 0.01 to 2 parts of citric acid, 0.01 to 2 parts of gluconolactone, 0.01 to 2 parts of calcium gluconate, 0.01 to 5 parts of hylocereus undatus fruit extract, 0.01 to 5 parts of phenoxyethanol, 0.01 to 5 parts of sodium pyrosulfite, 0.01 to 3 parts of leucojum aestivum bulb extract, 0.01 to 3 parts of olive leaf extract, 0.1 to 3 parts of nicotinamide, 0.01 to 2 parts of punica granatum fruit extract, 0.01 to 2 parts of dipalmitoyl hydroxyproline, and 0.001 to 0.5 part of eryngium maritimum callus culture filtrate. By virtue of the compounding of ingredients in the formula, the moisturizing cream provided by the invention has multiple efficacies such as moisturizing, blue light resistance, restoration,wrinkle resistance, whitening, speckle removal and the like.
Owner:蓓悠清(广东)健康科技有限公司
Steamed fruit cake and making method thereof
InactiveCN105309578AProtect NutrientsGreat tasteDough treatmentBakery productsFruit cakeVegetable oil
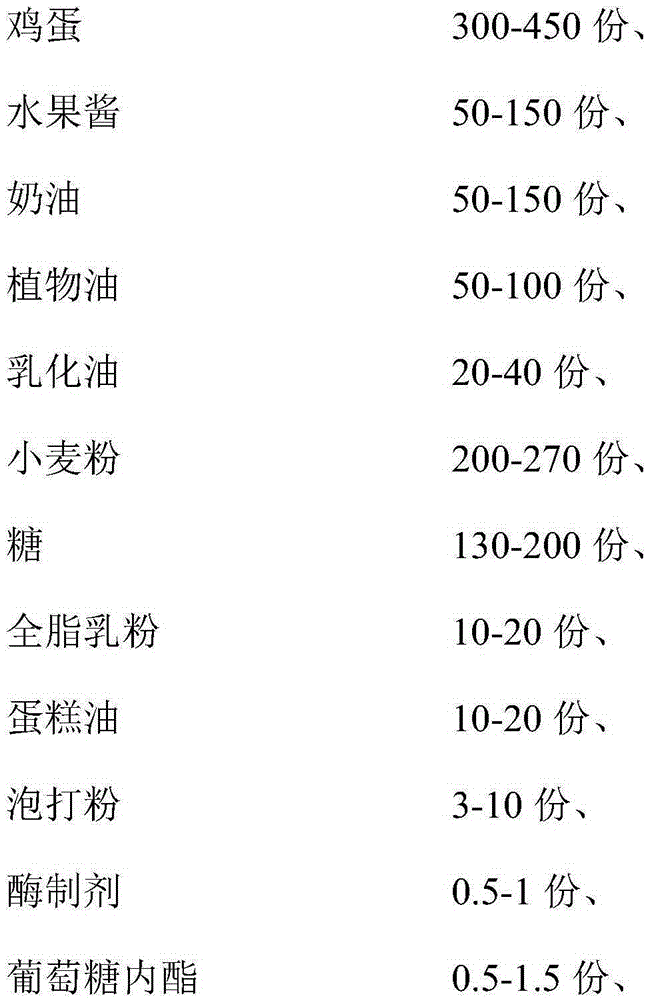
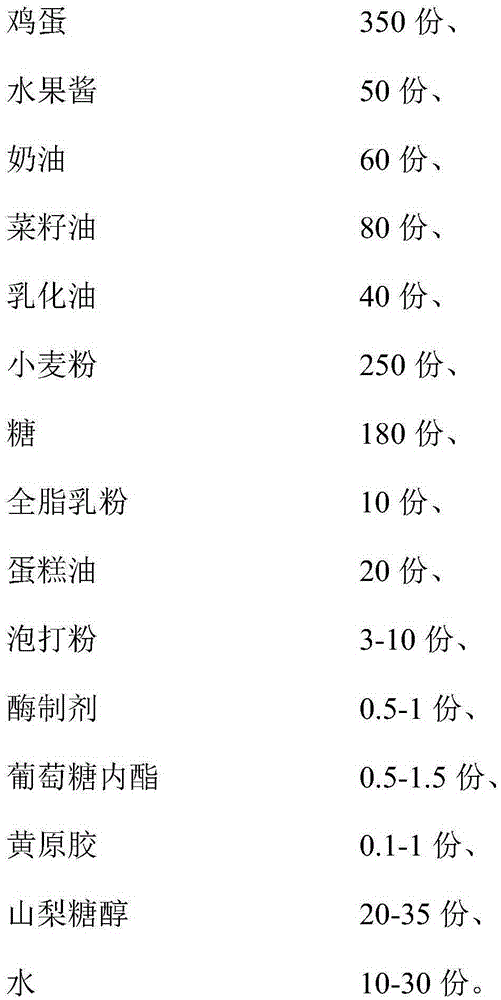
The invention discloses a steamed fruit cake. The steamed fruit cake is mainly made from, by weight, 300-450 parts of eggs, 50-150 parts of fruit jam, 50-150 parts of cream, 50-100 parts of vegetable oil, 20-40 parts of emulsified oil, 200-270 parts of sweat flour, 130-200 parts of sugar, 10-20 parts of whole milk powder, 10-20 parts of cake oil, 3-10 parts of baking powder, 0.5-1 part of an enzyme preparation, 0.5-1.5 parts of gluconolactone, 0.1-1 part of xanthan gum, 20-35 parts of sorbitol and 10-30 parts of water for standby application. According to the technical scheme of the steamed fruit cake, an improved formula is nutrient and healthy; meanwhile, a hot processing production method of twice paste injecting is adopted to prevent sinking of the sandwich fruit jam; through a technology of combining steaming with baking, the steamed fruit cake is fresh in mouthfeel, not only has the sweet and soft taste of a steamed cake, but also has the delicious, fine and smooth taste of a baked cake, can be stored for a longer time and is not prone to be hardened.
Owner:FUJIAN GUOLIANG FOOD CO LTD
Making process for Guanyin bean curd
InactiveCN104472727AAdjust the color at willSimple processing technologyCheese manufacturePremnaMass ratio
The invention provides a making process for Guanyin bean curd, and relates to the technical field of processing foods. The making process for the Guanyin bean curd comprises the following steps: (1), picking up 50-150 g of fresh tender leaves of adjoining premna of the year, and cleaning; (2), de-enzyming the tender leaves for 10-15 min by utilizing steam or hot wind at the temperature of 90-95 DEG C; (3), putting the tender leaves and water in a grinding machine according to the mass ratio of 1 to 5, and fully grinding the tender leaves and water; (4), filtering the ground semi-finished product by using a gauze, and obtaining a filtrate; (5), adding 0.5-1.5 g of 0.15% gel (including 80%-90% medical calcium carbonate and 10%-20% potassium bicarbonate) in the filtrate, simultaneously adding 0.01-1.5 g of 0.02% glucolactone, and stirring uniformly; (6) leaving the solution to stand for 1-2 hours to obtain the Guanyin bean curd. According to the making process for the Guanyin bean curd, the processing technique is simple, the operation is convenient, and the color of bean curd can be randomly regulated as required so as to increase the appetite of eaters.
Owner:ANHUI AGRICULTURAL UNIVERSITY
A kind of poultry egg tofu and preparation method thereof
The invention discloses poultry egg tofu and a production method thereof. It belongs to the field of food technology. Eggs and soybeans are used as the main raw materials, and gluconolactone is used as a coagulant. The parts by weight of each raw material are as follows: 98-100 parts of soybeans, 36-45 parts of eggs, 0.3-0.4 parts of gluconolactone, and defoaming agent 0.2-0.5 parts. The beneficial effects of the invention are: the bean curd made by the above method has fine and tender texture, pure taste, delicious taste, has dual nutritional components of eggs and soybeans, and is an indispensable food in people's daily life.
Owner:王兆彩
Manufacture method of bamboo shoot dietary fiber bean curds and product thereof
InactiveCN106720449ARaw materials are easy to getSimple processCheese manufactureFlavorDietary fiber
The present invention relates to a manufacture method of bamboo shoot dietary fiber bean curds and a product thereof. The preparation method is simple. By using wastes produced in the processing of bamboo shoots as a raw material and a variety of modern food high technologies, the bamboo shoot dietary fibers with a high purity are prepared by using compound enzymolysis, ultrafine grinding and other technologies, glucolactone is supplemented as a coagulator, and the bean curds containing the bamboo shoot dietary fibers are prepared. The prepared products are in square shapes (10cmx10cmx10cm), present milky white, smooth and beautiful in surface, uniform in color and luster, moderate in hardness, qualified in flexibility, and tender and smooth in taste, has unique flavor of the bamboo shoots, is free of other smell, and has good market development prospects.
Owner:SOUTHWEST UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com