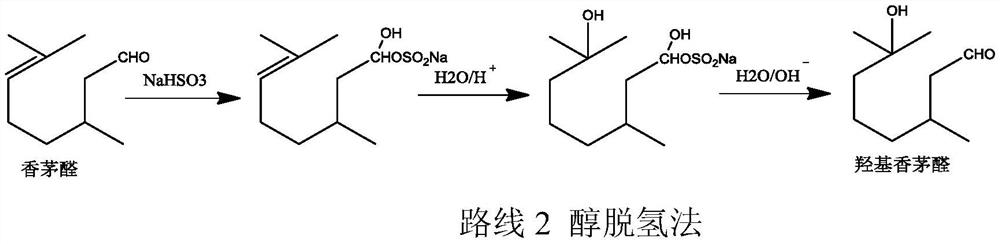
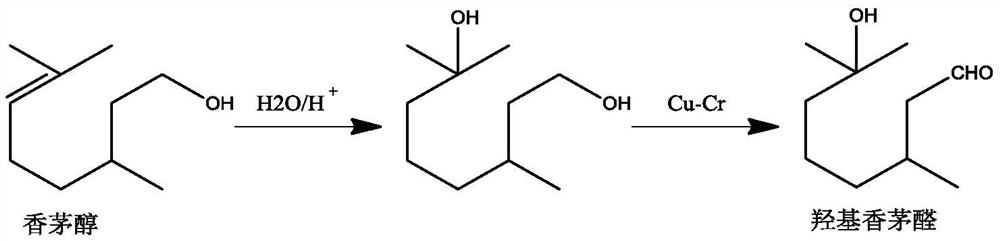
Method for preparing hydroxycitronellal through hydration reaction of citronellal
A technology for the hydration of hydroxycitronellal and malral, which is applied to the preparation of carbon-based compounds, the preparation of organic compounds, chemical instruments and methods, etc., to achieve the effects of simplified process flow, high selectivity, and reduced costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Pd / ZSM preparation:
[0065] 1) Preparation of Pd nano-sol
[0066] a) Take 10g polyvinylpyrrolidone, 5g Na 2 PdCl 4 Prepare a solution with 250g of water, and stir thoroughly at 10°C for 10h;
[0067] b) Take 3.86g NaBH 4 Add to the solution of a), fully stir at 10°C for 8h, then let it stand for 15h, and filter to obtain the nano-sol of metal Pd;
[0068] 2) Pd@SiO 2 preparation of
[0069] a1) Add 100g of ethanol and 10g of ammonia water (30wt%) into the metal Pd nano-sol, and fully stir for 5h at 10°C;
[0070] b1) Take 350.47 g of tetraethyl orthosilicate and add it to the mixture of a1), and fully stir at 10°C for 10 h;
[0071] c1) The mixture obtained in b1 was dried at 120°C for 15h to obtain 101.85g of SiO coated with Pd 2 , recorded as Pd@SiO 2 ;
[0072] 3) Preparation of Pd@ZSM
[0073] a2) Take 101.85g Pd@SiO respectively 2 , 1.44g AlOOH and 254.63g tetrapropylammonium hydroxide mixed and fully milled for 4h;
[0074] b2) Transfer the mixture ...
Embodiment 2
[0080] Pd / ZSM preparation:
[0081] 1) Preparation of Pd nano-sol
[0082] a) Take 20g polyvinylpyrrolidone, 5g Na 2 PdCl 4 Prepare a solution with 400g of water, and stir thoroughly for 1 hour at 15°C;
[0083] b) Take 2.58g NaBH 4 Add to the solution of a), fully stir at 25°C for 12h, then let it stand for 10h, and filter to obtain the nano-sol of metal Pd;
[0084] 2) Pd@SiO 2 preparation of
[0085] a1) Add 50g of ethanol and 20g of ammonia water (30wt%) into the metal Pd nano-sol, and fully stir for 1h at 15°C;
[0086] b1) Add 175.24 g of tetraethyl orthosilicate into the mixture of a1), and stir thoroughly at 30° C. for 15 h;
[0087] c1) The mixture obtained in b1 was dried at 110°C for 20 hours to obtain 51.81g of SiO coated with Pd 2 , recorded as Pd@SiO 2 ;
[0088] 3) Preparation of Pd@ZSM
[0089] a2) Take 51.81g Pd@SiO respectively 2 , 0.72g AlOOH and 233.15g tetrapropylammonium hydroxide were mixed and fully ground for 6h;
[0090] b2) transfer the ...
Embodiment 3
[0095] Pd / ZSM preparation:
[0096] 1) Preparation of Pd nano-sol
[0097] a) Take 30g polyvinylpyrrolidone, 5g Na 2 PdCl 4 Prepare a solution with 350g of water, and fully stir for 6 hours at 5°C;
[0098] b) Take 1.29g NaBH 4 Add to the solution of a), fully stir at 5°C for 2h, then let it stand for 20h, and filter to obtain the nano-sol of metal Pd;
[0099] 2) Pd@SiO 2 preparation of
[0100] a1) Add 150g of ethanol and 25g of ammonia water (30wt%) into the metal Pd nano-sol, and fully stir for 3h at 8°C;
[0101] b1) Add 35.05 g of tetraethyl orthosilicate to the mixture of a1), and stir thoroughly at 25°C for 13 hours;
[0102] c1) The mixture obtained in b1 was dried at 100°C for 10 h to obtain 11.78 g of Pd-coated SiO 2 , recorded as Pd@SiO 2 ;
[0103] 3) Preparation of Pd@ZSM
[0104] a2) Take 11.78g Pd@SiO respectively 2 , 0.13g AlOOH and 58.90g tetrapropylammonium hydroxide are mixed and fully ground for 1h;
[0105] b2) transfer the mixture obtained i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com