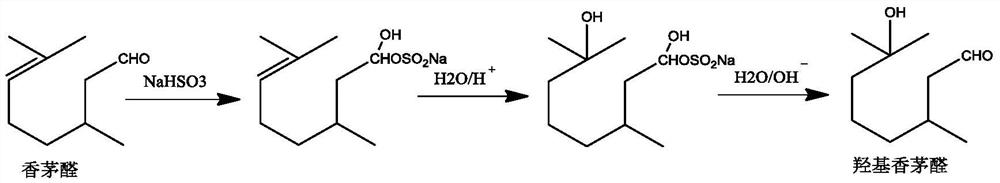
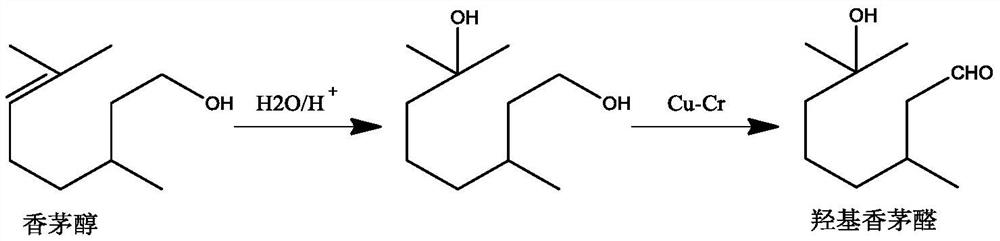
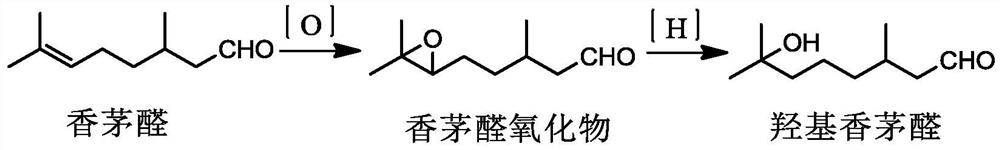
Method for preparing citronellal epoxide
A technology of epoxy and citronellal, which is applied in the direction of electrolysis process, electrolysis components, electrolysis organic production, etc., can solve the problems of easy deterioration, many by-products, poor stability of citronellal, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Mix 154g of raw material citronellal, 308g of deionized water, 77g of ethanol and 17.98g of (2-hydroxypropyl)-γ-cyclodextrin and use it as an electrolyte for later use, let it stand for 5 hours, and then transfer it to the electrolysis chamber with cathode and anode In the tank, use a Pt electrode for the anode and a Ni electrode for the cathode, the temperature is 15°C, and the current density is 0.1A / cm 2 The epoxidation of citronellal was carried out under certain conditions, and the electrochemical oxidation was completed after 4 hours of reaction.
[0049] Samples were taken for GC analysis, and the results are shown in Table 1.
Embodiment 2
[0051]Mix 154g of raw material citronellal, 462g of deionized water, 92.4g of ethanol and 53.94g of (2-hydroxypropyl)-γ-cyclodextrin as the electrolyte for later use, let it stand for 1h, and then transfer it to the In the electrolytic cell, a Pt electrode is used for the anode, and a Ni electrode is used for the cathode, the temperature is 20°C, and the current density is 0.3A / cm 2 The epoxidation of citronellal was carried out under the conditions, and the electrochemical oxidation was finished after 6 hours of reaction.
[0052] Samples were taken for GC analysis, and the results are shown in Table 1.
Embodiment 3
[0054] 154g of raw material citronellal, 616g of deionized water, 107.8g of ethanol and 89.90g of (2-hydroxypropyl)-γ-cyclodextrin were fully mixed as the electrolyte for later use. After standing for 10 hours, they were transferred to a In the electrolytic cell, a Pt electrode is used for the anode, and a Ni electrode is used for the cathode, the temperature is 10°C, and the current density is 0.15A / cm 2 The epoxidation of citronellal was carried out under certain conditions, and the electrochemical oxidation was completed after 2 hours of reaction.
[0055] Samples were taken for GC analysis, and the results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com