Nanometre complex solid superacid and preparation and application thereof
A solid super acid, nano-composite technology, applied in the preparation of carboxylate, the preparation of organic compounds, catalyst activation/preparation and other directions, can solve the problems of unfavorable industrialization promotion, high preparation cost, large specific surface area, etc., and achieve production cost. Low, good product quality, large specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
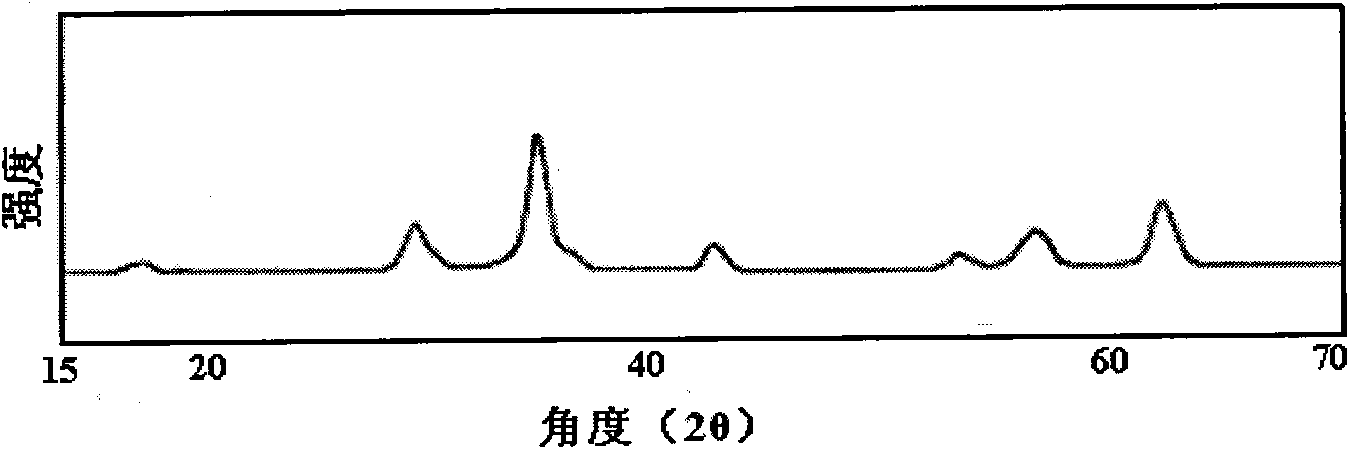
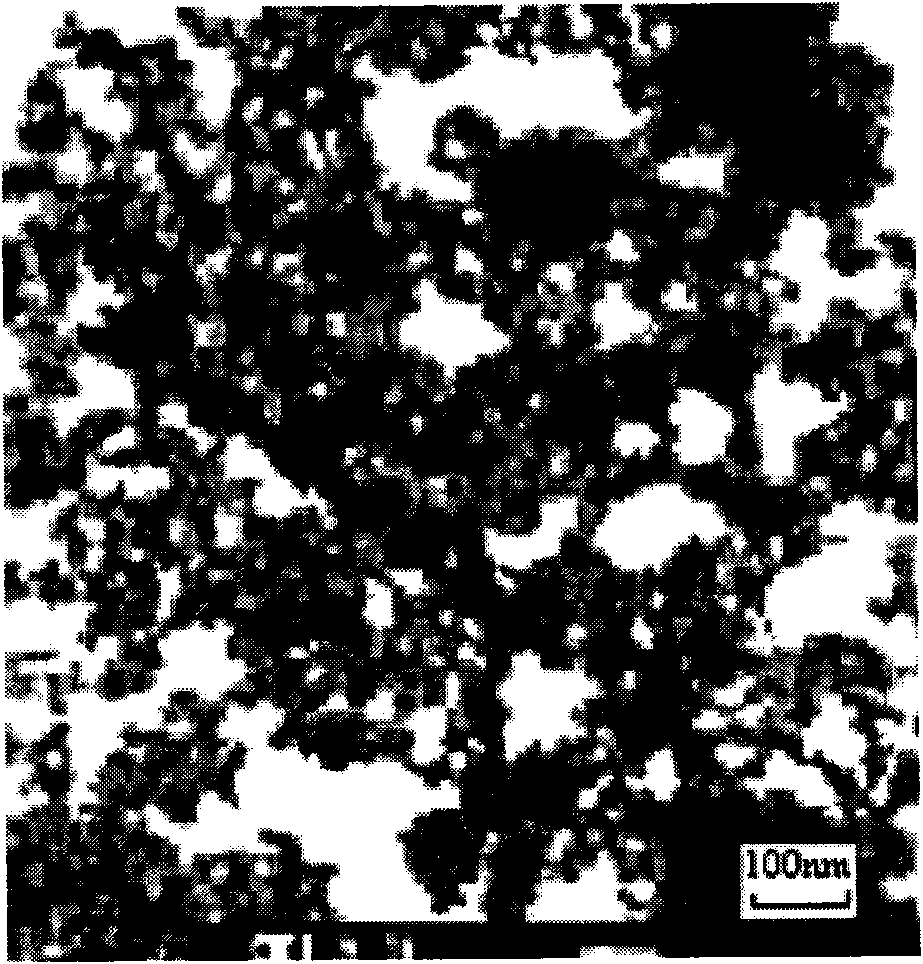
Image
Examples
Embodiment 1
[0027] a) press n (Fe 2+ ):n(Zn 2+ )=2.0:1 Weigh FeSO 4 ·7H 2 O and ZnSO 4 ·7H 2 O, mixed and ground into powder;
[0028] b) According to n(NaOH):n(Fe 2+ )=1.8:1 Add NaOH solution, and press n(PEG):n(Fe 2+ )=0.04:1 Add the dispersant polyethylene glycol (PEG-600), and stir at 50-70°C for 30-60 minutes;
[0029] c) Press n (NaHCO 3 ):n(Fe 2+ )=2.0:1 adding NaHCO 3 Solid, adjust pH=10, stir at 50-70°C for 10-20 minutes;
[0030] d) Aging at 20-40°C for 10 hours, suction filtering, and washing the obtained precipitate with absolute ethanol until neutral;
[0031] e) Infrared drying the precipitate at 90-100°C for 3-10 hours to obtain nano-zinc ferrite (ZnFe 2 o 4 )Precursor;
[0032] f) Naturally cool the precursor to room temperature, grind it into powder, and then impregnate it in 0.75mol / L (NH 4 ) 2 S 2 o 8 In the solution, after soaking for 10 hours, filter, and dry the obtained solid at 110°C for 6 hours;
[0033] g) Roasting at 500K for 3 hours, annealin...
Embodiment 2
[0040] a) press n (Fe 2+ ):n(Zn 2+ )=1.8:1 Weigh FeSO 4 ·7H 2 O and ZnSO 4 ·7H 2 O, mixed and ground into powder;
[0041] b) According to n(NaOH):n(Fe 2+ )=1.6:1 Add NaOH solution, and press n(PEG):n(Fe 2+ )=0.02:1 Add the dispersant polyethylene glycol (PEG-600), and stir at 50-70°C for 30-60 minutes;
[0042] c) Press n (NaHCO 3 ):n(Fe 2+ )=1.8:1 adding NaHCO 3 Solid, adjust pH=11, stir at 50-70°C for 10-20 minutes;
[0043] d) Aging at 20-40°C for 7 hours, suction filtering, and washing the obtained precipitate with absolute ethanol until neutral;
[0044] e) Infrared drying the precipitate at 90-100°C for 3-10 hours to obtain nano-zinc ferrite (ZnFe 2 o 4 )Precursor;
[0045] f) The precursor is naturally cooled to room temperature, ground into powder, and then impregnated in 1.2mol / L (NH 4 ) 2 S 2 o 8 In the solution, filter after soaking for 6 hours, and dry the obtained solid at 100°C for 8 hours;
[0046] g) Roasting at 550K for 3 hours, annealing a...
Embodiment 3
[0053] a) press n (Fe 2+ ):n(Zn 2+ )=1.5:1 Weigh FeSO 4 ·7H 2 O and ZnSO 4 ·7H 2 O, mixed and ground into powder;
[0054] b) According to n(NaOH):n(Fe 2+ )=1.5:1 NaOH solution was added, and n(PEG):n(Fe 2+ )=0.06:1 Add the dispersant polyethylene glycol (PEG-600), and stir at 50-70°C for 30-60 minutes;
[0055] c) Press n (NaHCO 3 ):n(Fe 2+ )=1.7:1 adding NaHCO 3 Solid, adjust pH=8, stir at 50-70°C for 10-20 minutes;
[0056] d) Aging at 20-40°C for 3 hours, suction filtering, and washing the obtained precipitate with absolute ethanol until neutral;
[0057] e) Infrared drying the precipitate at 90-100°C for 3-10 hours to obtain nano-zinc ferrite (ZnFe 2 o 4 )Precursor;
[0058] f) The precursor is naturally cooled to room temperature, ground into powder, and then impregnated in 0.5mol / L (NH 4 ) 2 S 2 o 8 In the solution, after soaking for 12 hours, filter, and dry the obtained solid at 90°C for 10 hours by infrared;
[0059] g) Roasting at 450K for 3 hours...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com