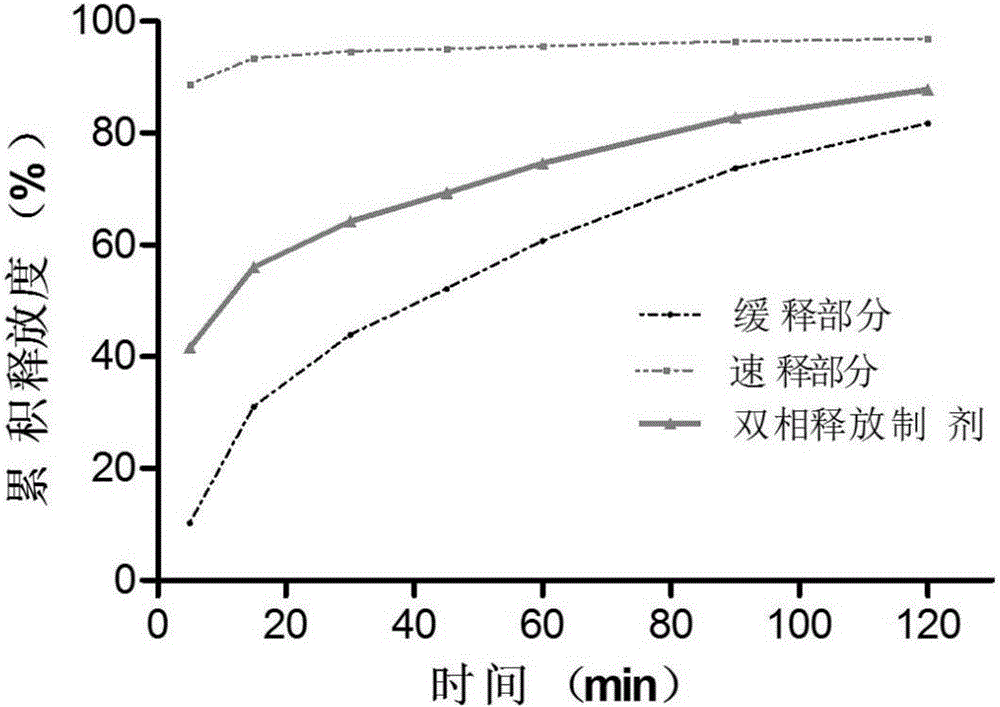
Racecadotril double-phase releasing preparation and preparation method thereof
A technology of racecadotril and racecadotril, which is applied in medical preparations containing active ingredients, medical preparations without active ingredients, and digestive system, etc. Exceeding the standard and high equipment requirements, to achieve the effects of low equipment requirements, improved dissolution, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] prescription
[0027] (1) Prescription composition of drug-containing granules
[0028]
[0029]
[0030] The preparation process and process of drug-containing granules are as follows: crush the drug and sucrose to a certain particle size range, mix the drug, sucrose, ethyl cellulose or hypromellose in a wet granulator for 5 minutes; add purified water to prepare Soft material; then put the soft material into a 40-mesh sieve of a swinging granulator to granulate; the formed granules are dried in a vacuum drying oven at 50±5°C until the moisture content is less than 1%, and then granulated with a 40-mesh sieve to obtain .
[0031] (2) Composition of racecadotril biphasic drug release preparation
[0032]
[0033] Put the sustained-release drug-containing granules and quick-release drug-containing granules, peach flavor essence, and fumed silicon dioxide in a mixer according to the above-mentioned proportions and mix until uniform, and divide into bags so that...
Embodiment 2
[0035] prescription
[0036] (1) Prescription composition of drug-containing granules
[0037]
[0038]
[0039] The preparation process and process of drug-containing granules are as follows: crush the drug and sucrose to a certain particle size range, mix the drug, sucrose, ethyl cellulose or hypromellose in a wet granulator for 5 minutes; add purified water to prepare Soft material; then put the soft material into a 40-mesh sieve of a swinging granulator to granulate; the formed granules are dried in a vacuum drying oven at 50±5°C until the moisture content is less than 1%, and then granulated with a 40-mesh sieve to obtain .
[0040] (2) Composition of racecadotril biphasic drug release preparation
[0041]
[0042] Put the sustained-release drug-containing granules and quick-release drug-containing granules, peach flavor essence, and fumed silicon dioxide in a mixer according to the above-mentioned proportions and mix until uniform, and divide into bags so that...
Embodiment 3
[0044] prescription
[0045] (1) Prescription composition of drug-containing granules
[0046]
[0047]The preparation process and process of drug-containing granules are as follows: crush the drug and sucrose to a certain particle size range, mix the drug, sucrose, ethyl cellulose or hypromellose in a wet granulator for 5 minutes; add purified water to prepare Soft material; then put the soft material into a 40-mesh sieve of a swinging granulator to granulate; the formed granules are dried in a vacuum drying oven at 50±5°C until the moisture content is less than 1%, and then granulated with a 40-mesh sieve to obtain .
[0048] (2) Composition of racecadotril biphasic drug release preparation
[0049]
[0050] Put the sustained-release drug-containing granules and quick-release drug-containing granules, peach flavor essence, and fumed silicon dioxide in a mixer according to the above-mentioned proportions and mix until uniform, and divide into bags so that each bag con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com